Surface Chemistry - Preparation of Colloids | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Preparation of Colloids
Preparation of Colloids
Many lyophillic substances are made in their colloidal form by warming
with water. Rubber forms colloidal solution with benzene. Soap spontaneously
forms a colloidal solution by just mixing with water.
In general, colloidal are prepared by the following methods.
i. Dispersion
methods: In this method larger particles are broken to colloidal dimension.
ii. Condensation
method: In this method, smaller atom or molecules are converted into larger
colloidal sized particles.
1) Dispersion methods
(i) Mechanical Dispersion:
Using a colloid mill, the solid is ground to colloidal dimension. The
colloid mill consists of two metal plates rotating in opposite direction at
very high speed of nearly 7000 revolution / minute.
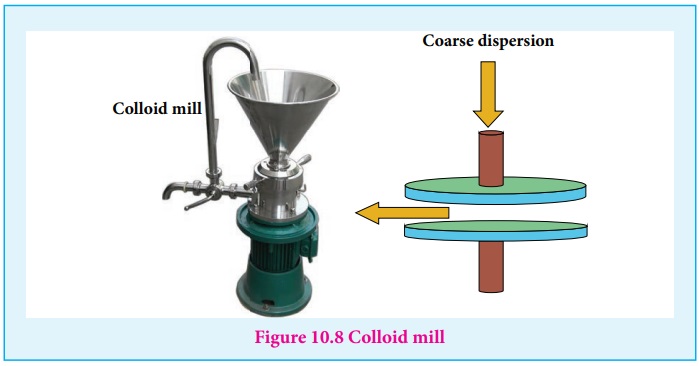
The colloidal particles of required colloidal size is obtained by
adjusting the distance between two plates.
By this method, colloidal solutions of ink and graphite are prepared.
(ii) Electro Dispersion:
A brown colloidal solution of platinum was first prepared by George Bredig in 1898. An electrical arc is struck between electrodes dispersed in water surrounded by ice. When a current of 1 amp /100 V is passed an arc produced forms vapours of metal which immediately condense to form colloidal solution. By this method colloidal solution of many metals like copper, silver, gold, platinum, etc. can be prepared Alkali hydroxide is added as an stabilising agent for the colloidal solution.
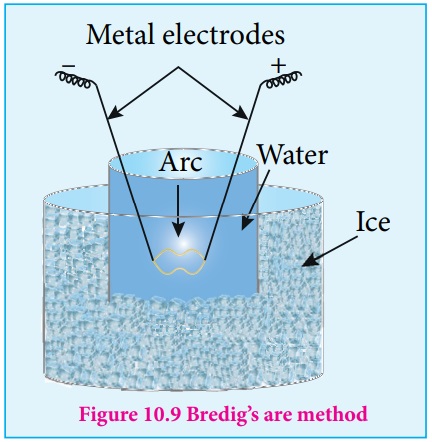
Svedberg modified this method for the preparation of non aqueous
inflammable liquids like pentane, ether and benzene, etc using high frequency
alternating current which prevents the decomposition of liquid.
(iii) Ultrasonic dispersion
Sound waves of frequency more than 20kHz (audible limit) could cause
transformation of coarse suspension to colloidal dimensions.
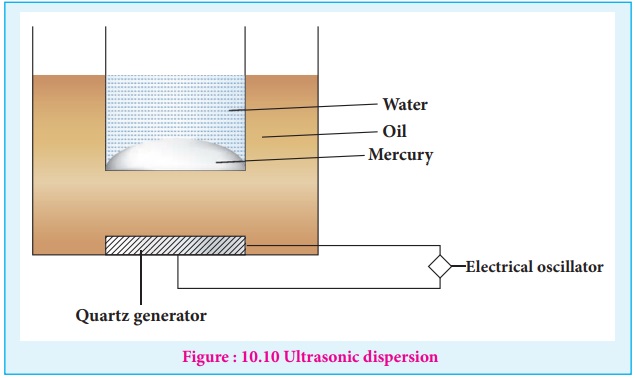
Claus obtained mercury sol by subjecting mercury to sufficiently high
frequency ultrasonic vibrations.
The ultrasonic vibrations produced by generator spread the oil and
transfer the vibration to the vessel with mercury in water.
(iv) Peptisation:
By addition of suitable electrolytes, precipitated particles can be
brought into colloidal state. The process is termed as peptisation and the
electrolyte added is called peptising or dispersing agent.
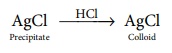
AgCl …HCL→ AgCl
Precipitate …HCL→ Colloid
2) Condensation Methods:
When the substance for colloidal particle is present as small sized
particle, molecule or ion, they are brought to the colloidal dimension by
condensation methods. Here care should be taken to produce the particle with
colloidal size otherwise precipitation will occur. Various chemical methods for
the formation of colloidal particles.
(i) Oxidation:
Sols of some non metals are prepared by this method.
(a) When hydroiodic acid is treated with iodic acid, I2 sol
is obtained.
HIO3 +5HI → 3H 2O
+ I2 (Sol)
(b) When O2 is passed through H2Se, a sol of
selenium is obtained.
H 2 Se + O2 → 2H2 O + Se(sol)
(ii) Reduction:
Many organic reagents like phenyl hydrazine, formaldehyde, etc are used
for the formation of sols. For example: Gold sol is prepared by reduction of
auric chloride using formaldehyde.
2AuCl 3 +3HCHO+3H2O → 2Au(sol)+6HCl+3HCOOH
(iii) Hydrolysis
Sols of hydroxides of metals like chromium and aluminium can be produced
by this method.
For
Example,
FeCl3 +3H 2 O → Fe(OH)3 +3HCl
(iv) Double decomposition
For the preparation of water insoluble sols this method can be used.
When hydrogen sulphide gas is passed through a solution of arsenic
oxide, a yellow coloured arsenic sulphide is obtained as a colloidal solution.
As2 O 3 + 3H2 S → As 2
S3 +3H 2 O
(v) Decomposition
When few drops of an acid is added to a dilute solution of sodium
thiosulphate, the insoluble free sulphur produced by decomposition of sodium
thiosulphate accumulates into small, clusters which impart various colours
blue, yellow and even red to the system depending on their growth within the
size of colloidal dimensions.
S2O32-+2H+ → S (sol) + H2O + SO2
3) By exchange of solvent:
Colloidal solution of few substances like phosphorous or sulphur is
obtained by preparing the solutions in alcohol and pouring them into water. As
they are insoluble in water, they form colloidal solution.
P in alcohol + water→ Psol.
Related Topics