Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Various application of colloids
Various application of colloids
In every path of life, colloids play a great role. Human body contains
the numerous colloidal solutions. The blood in our body, protoplasma of plant
and animal cell, and fats in our intestines are in the form of emulsions.
Synthetic polymers like polystyrene silicones and PVC are colloids.
Food
Food stuffs like milk cream, butter, etc are present in colloidal form.
Medicines
Antibodies such as penicillin and streptomycin are produced in colloidal
form for suitable injections. Colloidal gold and colloidal calcium are used as
tonics. Milk of magnesia is used for stomach troubles. Silver sol protected by
gelatine known as Argyrol is used as eye lotion.
In Industry
Colloids find many applications in industries.
(i) Water purification:
Purification of drinking water is activated by coagulation of suspended
impurities in water using alums containing Al3+
(ii) In washing:
The cleansing action of soap is due to the formation of emulsion of soap
molecules with dirt and grease.
(iii) Tanning of leather
Skin and hides are protein containing positively charged particles which
are coagulated by adding tannin to give hardened leather for further
application. Chromium salts are used for the purpose. Chrome tanning can
produce soft and polishable leather.
(iv) Rubber industry:
Latex is the emulsion of natural rubber with negative particles. By
heating rubber with sulphur, vulcanized rubbers are produced for tyres, tubes,
etc.
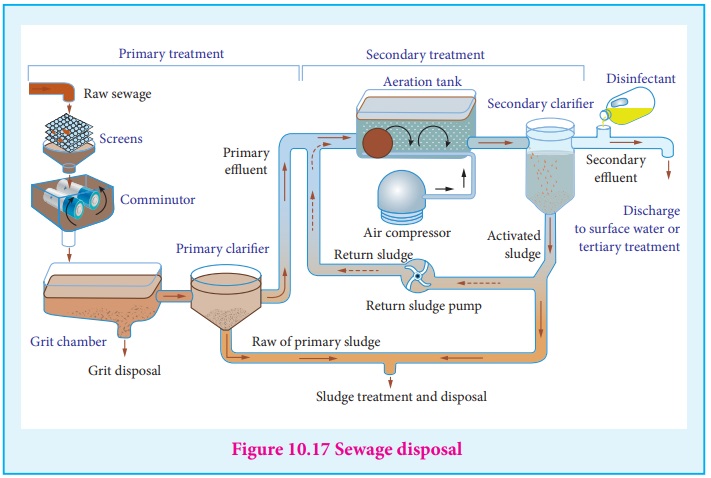
(v) Sewage disposal
Sewage contains dirt, mud and wastes dispersed in water. The passage of
electric current deposits the wastes materials which can be used as a manure.
(vi ) Cortrell’s precipitator
Carbon dust in air is solidified by cortrell’s precipitator. In it, a
high potential difference of about 50,000V is used. The charge on carbon is
neutralized and solidified. Thus the air is free from carbon particles.
Vii) The blue colour of the sky in nature is
due to Tyndall effect of air particles.
viii) Formation of delta:
The electrolyte in sea and river water coagulates the solid particles in
river water at their intersection. So, the earth becomes a fertile land.
Ix) Analytical application
Qualitative and quantitative analysis are based on the various
properties of colloids.
Hence we can conclude that in our life, there is hardly any field which
is not including the applications of colloids.
Natural honey is a colloidal sol. It is distinguished from
artificial one by adding ammoniacal AgNO3
In case of natural honey a metallic silver is produced, assumes
a reddish yellow color due to traces of albumin or ethereal oil which acts as a
protective colloid. In case of artificial honey a dark yellow or greenish
yellow precipitate is formed.
Related Topics