Surface Chemistry - Choose the correct answer | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Choose the correct answer
Chemistry : Surface Chemistry
EVALUATION
Choose the correct answer:
1. For Freundlich isotherm a graph of log (x/m) is plotted against log p. The slope of the line and its y – axis intercept respectively corresponds to
a) 1/n , k
b) log 1/n , k
c) 1/n , log k
d) log 1/n , log k
Solution:
x/m =k.p1/n
log(x/m )=logk+ 1/ n logp
y=c+mx
m = 1/n and c= logk
option: (c)
2. Which of the following is incorrect for physisorption?
a) reversible
b) increases with increase in temperature
c) low heat of adsorption
d) increases with increase in surface area
Solution:
The incorrect statement is option (b)
Physisorption is an exothermic process. Hence increase in temperature decreases the physisorption.
3. Which one of the following characteristics are associated with adsorption?
a) ∆G and ∆H are negative but ∆S is positive
b) ∆G and ∆S are negative but ∆H is positive
c) ∆G is negative but ∆H and ∆S are positive
d) ∆G, ∆H and ∆S all are negative.
Solution:
Adsorption leads to decrease in randomness (entropy).i.e. ∆S< 0 for the adsorption to occur, ∆G should be -ve. We know that ∆G=∆H-T∆S if ∆S is -ve, T∆S is +ve. It means that ∆G will become negative only when ∆H is -ve and ∆H>T∆S
Solution: Option (d)
4. Fog is colloidal solution of
a) solid in gas
b) gas in gas
c) liquid in gas
d) gas in liquid
Solution:
dispersion medium-gas dispersed phase-liquid
Solution: Option (c)
5. Assertion : Coagulation power of Al3+ is more than Na+ .
Reason : greater the valency of the flocculating ion added, greater is its power to cause precipitation
a) if both assertion and reason are true and reason is the correct explanation of assertion.
b) if both assertion and reason are true but reason is not the correct explanation of assertion.
c) assertion is true but reason is false
d) both assertion and reason are false.
Solution:
Option (a) (Hardy-Schulze rule)
6. Statement : To stop bleeding from an injury, ferric chloride can be applied. Which comment about the statement is justified?
a) It is not true, ferric chloride is a poison.
b) It is true, Fe3+ ions coagulate blood which is a negatively charged sol
c) It is not true; ferric chloride is ionic and gets into the blood stream.
d) It is true, coagulation takes place because of formation of negatively charged sol with Cl-.
Solution: Option (b)
7. Hair cream is
a) gel
b) emulsion
c) solid sol
d) sol.
Solution:
Emulsion dispersed phase
Dispersion medium -liquid
8. Which one of the following is correctly matched?
a) Emulsion – Smoke
b) Gel – butter
c) foam – Mist
d) whipped cream – sol
Solution: Option (b)
9. The most effective electrolyte for the coagulation of As2 S3Sol is
a) NaCl
b) Ba(NO3 )2
c) K3 [Fe(CN)6]
d) Al2(SO4)3
Solution:
As2S3 is a -vely charged colloid. It will be most effectively coagulated by the cation with greater valency. i.e., Al3+ .
Solution: Option (d)
10. Which one of the is not a surfactant?
a) CH3 --- (CH2)15 --- +N –(--CH3)2 CH2 Br
b) CH3 --- (CH2)15 --- NH2
c) CH3 --(-- CH2 --)16-- CH2 OSO2-- Na+
d) OHC --(--CH2--)14 -- CH2 – COO-- Na+
Solution: Option (b)
11. The phenomenon observed when a beam of light is passed through a colloidal solution is
a) Cataphoresis
b) Electrophoresis
c) Coagulation
d) Tyndall effect
Solution: Tyndall effect-scattering of light
Option (d)
12. In an electrical field, the particles of a colloidal system move towards cathode. The coagulation of the same sol is studied using K2 SO4 (i), Na3 PO4 (ii),K4 [Fe(CN)6 ] (iii) and NaCl (iv) Their coagulating power should be
a) II > I>IV > III
b) III > II > I > IV
c) I > II > III > IV
d) none of these
Solution: Option (b)
13. Collodion is a 4% solution of which one of the following compounds in alcohol – ether mixture?
a) Nitroglycerine
b) Cellulose acetate
c) Glycoldinitrate
d) Nitrocellulose
Solution: pyroxylin(nitro cellulose)
Solution: Option (d)
14. Which one of the following is an example for homogeneous catalysis?
a) manufacture of ammonia by Haber’s process
b) manufacture of sulphuric acid by contact process
c) hydrogenation of oil
d) Hydrolysis of sucrose in presence of dil HCl
Solution: Both reactant and catalyst are in same phase. i.e(l)
Solution: Option (d)
15. Match the following
A) V2O5 i) High density polyethylene
B) Ziegler – Natta ii) PAN
C) Peroxide iii) NH3
D) Finely divided Fe iv) H2 SO4
A B C D
a) (iv) (i) (ii) (iii)
b) (i) (ii) (iv) (iii)
c) (ii) (iii) (iv) (i)
d) (iii) (iv) (ii) (i)
Solution: Option (a)
16. The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As2S3 are given below
(I) (NaCl)=52 (II) ((BaCl2 )=0.69 (III) (MgSO4 )=0.22
The correct order of their coagulating power is
a) III > II > I
b) I > II > III
c) I > III > II
d) II > III>I
Solution: coagulating power α = 1/ coagulation value
Solution: Option (a)
17. Adsorption of a gas on solid metal surface is spontaneous and exothermic, then
a) ∆H increases
b) ∆S increases
c) ∆G increases
d) ∆S decreases
Solution: ∆S is –ve
Solution: Option (d)
18. If x is the amount of adsorbate and m is the amount of adsorbent, which of the following relations is not related to adsorption process?
a) x/m = f(P) at constant T
b) x/m = f(T) at constant P
c) P = f(T) at constant x/m
d) x/m = PT
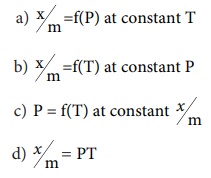
Solution: Option (d)
19. On which of the following properties does the coagulating power of an ion depend ?
a) Both magnitude and sign of the charge on the ion.
b) Size of the ion alone
c) the magnitude of the charge on the ion alone
d) the sign of charge on the ion alone.
Solution: Option (a)
20. Match the following
A) Pure nitrogen i) Chlorine
B) Haber process ii) Sulphuric acid
C) Contact process iii) Ammonia
D) Deacons Process iv) sodium azide (or) Barium azide
Which of the following is the correct option?
A B C D
a) (i) (ii) (iii) (iv)
b) (ii) (iv) (i) (iii)
c) (iii) (iv) (ii) (i)
d) (iv) (iii) (ii) (i)
Solution: Option (d)
PTA Questions and Answers
Which of the following correctly matched ?
a) Emulsion - Paint
b) Liquid Aerosol
c)
Foam - Pumice stone
d) Gel - Butter
Answer: d)
2. Which one of the following is
negatively charged colloid?
a) arsenic sulphide
b)
Ferric hydroxide
c)
Haemoglobin
d)
Basic dyes
Answer: a)
3. The change of W/O emulsion in
to O/W emusion is called ______
a)
Coagulation
b)
Emulsification
c)
Decompositon
d)
Inversion of phase
Answer: a)
4. The blue colour of water in
the sea is due to
a) Scattering of blue light by
water molecules
b)
Reflection of blue sky by sea water
c)
Refraction of blue light by the impurities in sea water
d)
Adsorption of other colours, except the blue colour by water molecules
Answer: a)
Related Topics