Surface Chemistry - Short Question Answer | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Short Question Answer
Surface Chemistry
Short Answer
1. Give two important characteristics of physiscorption
1.
It is instantaneous and non specific
2.
When pressure increases, physisorption also increases
3.
Multilayer of adsorbate formed
2. Differentiate physisorption and chemisorption

Physical adsorption or van der
waals adsorption or Physisorption
1.
It is instantaneous
2.
It is non-specific
3.
Pressure increases the amount of adsorption increases.
4.
Decreases with increase in temperature.
5.
Heat of adsorption is low
6.
Multilayer
7.
Occurs on all sides.
Chemical adsorption or
Chemisorption or Activated adsorption
1.
It is very slow
2.
It is specific
3.
Pressure can not alter.
4.
When temperature is raised first increases and then decreases.
5.
Heat of adsorption is high
6.
Monolayer
7.
Occurs at fixed sites
3. In case of chemisorption, why adsorption first increases and then decreases with temperature?
• Increase in temperature will
provide the molecule necessary activation energy for chemical bond formation
hence the rate of adsorption increases.
• At a certain temperature all
bonds are formed and now further increase in temperature will favours
desorption.
4. Which will be adsorbed more readily on the surface of charcoal and why? NH3 or O2 ?
• The critical temperature of
ammonia gas is quite higher than carbon dioxide.
• Therefore it is easily combines
with the surface of charcoal.
• Therefore NH3 will be
more readily adsorbed on the surface of the charcoal than CO2.
5. Heat of adsorption is greater for chemisorptions than physisorption. Why?
• In adsorbed state the adsorbate
is held on the surface of the adsorbent by strong chemical bond and it is much
stronger than physisorption.
• Hence heat of adsorption is
greater.
6. In a coagulation
experiment 10 mL of a colloid (X) is mixed with distilled water and 0.01M solution
of an electrolyte AB so that the volume is 20 mL. It was found that all
solutions containing more than 6.6 mL of AB coagulate with in 5 minutes. What
is the flocculation values of AB for sol (X)?
Answer:
A
minimum of 6.6mL of AB is required to coagulate the sol. The moles of AB in the
sol is
(6.6
× 0.01) / 20 = 0.0033 moles
This
means that a minimum of 0.0033 moles or 0.0033 × 1000 = 3.3 milli moles are
required for coagulating 1 litre of sol.
Flocculation
value of AB for X = 3.3
7. Peptising agent is added to convert precipitate into colloidal solution. Explain with an example.
• Addition of suitable electrolytes
to the precipitated particles give colloidal solution.
• The process is termed as
peptisation and the electrolyte added is called peptising agent.
Example:
AgCl ___HCl→ AgCl
Precipitate Colloid
AgCl
precipitate is converted to AgCl colloid by adding an peptising agent HCl.
8. What happens when a colloidal sol of Fe(OH)3 and As2S3 are mixed?
• Fe(OH)3 is positively
charged colloid whereas AS2O3 is negatively charged
colloid.
• Coagulation takes place when the
positively charged Fe(OH)3 is mixed with negatively charged AS2O3.
9. What is the difference between a sol and a gel?
Sol: Liquid
in solid is called sol
Example:
Inks, colloidal gold
Gel: Solid in
liquid is called gel
Example:
Butter, cheese
10. Why are lyophillic colloidal sols are more stable than lyophobic colloidal sol.
• Due to solvation the interaction
between the dispersed phase and dispersed medium is strong in the lyophillic
colloidal sols.
• In lyophobic sol the stability is
due to charge. The interaction between the dispersed phase and dispersion
medium is very little. Hence lyophillic colloidal sols are more stable than
lyophobic colloidal sol.
11. Addition of Alum purifies water. Why?
Addition
of alum coagulate the suspended impurities in water and the drinking water is
purified.
12. What are the factors which influence the adsorption of a gas on a solid?
i)
Nature of adsorbent
ii)
Nature of adsorbate
iii)
Pressure
iv)
Temperature
i. Nature of adsorbent
• Higher the surface area of
adsorbent, higher is the amount adsorbed.
ii. Nature of adsorbate
• Due to greater van der waal’s
force of attraction easily liquefiable gases are adsorbed readily. Eg: CO2,
NH3
• Permanent gases are having low
critical temperature, can not be liquefied easily and adsorbed slowly. Eg. H2,
N2 and O2
iii. Effect of Pressure
• In Physisorption, when pressure
increases the amount of adsorption increases.
• Pressure can not alter the amount
of chemisorption.
iv. Effect of Temperature
• Physisorption decreases with
increase in temperature.
• When temperature is raised
chemisorption first increases and then decreases
13. What are enzymes? Write a brief note on the mechanism of enzyme catalysis.
Enzymes
are complex protein molecules with three dimensional structures. It catalyse the
chemical reaction in living organism.
The
following mechanism is proposed for the enzyme catalysis
E
+ S ⇌ ES → P + E
Where
E is the enzyme
S
is the substrate (reactant)
ES
is activated complex
P
is the product.
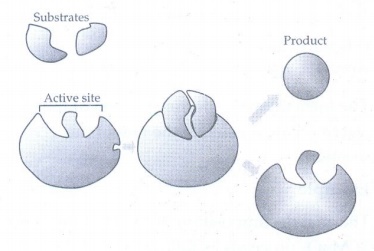
14. What do you mean by activity and selectivity of catalyst?
Activity: The
reactants must get adsorbed strongly on the catalyst to become active. The activity
depends on the strength of chemisorption.
Selectivity: it is
ability of a catalyst to direct a reaction to yield a particular product
selectively.
15. Describe some feature of catalysis by Zeolites.
• Zeolites are microporous,
crystalline, hydrated, alumino silicates
• Extra cations are present in the
framework, to balance the extra negative charge.
• It is shape selective.
•
Reactant shape selectivity: When
bulkier molecules in a reactant mixture are prevented from reaching the active
sites within the zeolite crystal
•
Transition state selectivity: If the
transition state of a reaction is large compared to the pore size of the
zeolite, then no product will be formed.
•
Product selectivity: It is
encountered when certain product molecules one too big to diffuse out of the
zeolite pores
• Zeolites carrying protons are
used in the petrochemical industry
Zeolites
carring Na+ ions are used as catalysis
16. Give three uses of emulsions.
1.
Food stuffs like milk cream, butter, etc are present in colloidal form
2.
The cleansing action of soap is due to the formation of emulsion of soap
molecules with dirt and grease
3.
Latex is the emulsion of natural rubber with negative particles. By heating
rubber with sulphur, vulcanized rubbers are produced for tyres, tubes
17. Why does bleeding stop by rubbing moist alum
Blood
is a colloidal form. When alum is rubbed to the bleeding part of the body the
coagulation of blood takes place and the bleeding is arrested.
18. Why is desorption important for a substance to act as good catalyst?
• The formed product should leave
from the surface of the catalyst to create free surface for other reactant
molecule.
• If the desorption does not occur
then the other reactants are left with no space on the surface of the catalyst
and the yield will be poor.
• Hence desorption is important for
a substance to act as good catalyst.
19. Comment on the statement: Colloid is not a substance but it is a state of substance.
The
size of the colloidal particle is between 1 - 200 nm. Any particle is present
between this size are behaves as colloid. Hence it is a state of substance and
not a substance.
20. Explain any one method for coagulation
By
mixing two oppositively charged sol:
When
colloidal sols with opposite charges are mixed mutual coagulation takes place.
It is due to migration of ions from the surface of the particles
21. Write a note on electro osmosis
The
movement of dispersion medium under the influence of electric potential is
called electro osmosis.
A
sol is electrically neutral. The medium carries an equal but opposite charge to
that of dispersed particles.
Under
the influence of electric field the medium moves in a direction opposite to
that of the sol particles.
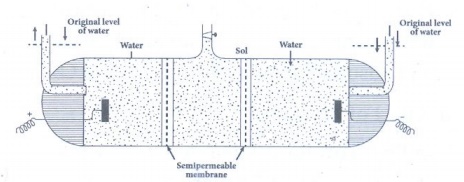
22. Write a note on catalytic poison
• The substances which decreases or
completely destroys the activity of catalyst in a catalysed reaction are known
as catalytic poisons.
• In the Haber's process, the Fe
catalyst is poisoned by H2S.

23. Explain intermediate compound formation theory of catalysis with an example
i)
In homogeneous catalysed reactions a catalyst may combine with one or more
reactant to form an intermediate
ii)
Activation energy for the intermediate formation is lower than product formation
iii)
The intermediate reacts with other reactant or decompose to give products and
the catalyst is regenerated.
Consider
the general reaction:
A+B
→ AB (1)
A+C
→ AC (intermediate) (2)
C
is the catalyst
AC+B
→ AB+C (3)
The
formation and decomposition of the intermediate accelerate the rate of the
reaction.
Example
Thermal
decomposition of KClO3 in presence of MnO2 proceeds as
follows.
2KClO3
→ 2KCl+3O2
Mechanism
2KClO3
+ 6MnO2 → 6MnO3 + 2KCl
MnO3
is an intermediate.
6MnO3
→ 6MnCO2 +3O2
Catalyst
MnO2 is regenerated
24. What is the difference between homogenous and hetrogenous catalysis?
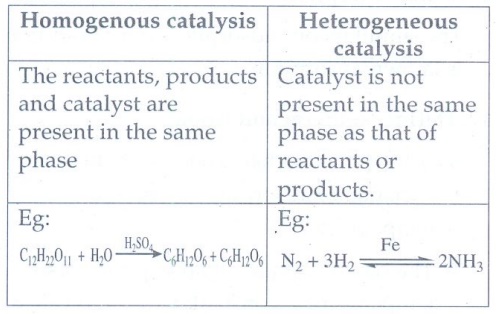
Homogenous catalysis
The
reactants, products and catalyst are present in the same phase
Eg:
C12H22O11 + H2O ___H2SO4__→ C6H12O6
+ C6H12O6
Heterogeneous catalysis
Catalyst
is not present in the same phase as that of reactants or products.
Eg:
N2 + 3H2 ←__Fe_→ 2NH3
25. Describe adsorption theory of catalysis.
Adsorption
theory also called as contact catalysis.
Langmuir
explained the various steps involved in a heterogeneous catalysed reaction.
1.
Reactant molecules diffuse from bulk to the catalyst surface.
2.
The reactant molecules are adsorbed on the surface of the catalyst.
3.
The adsorbed reactant molecules are activated and form activated complex which
is decomposed to form the products
4.
The product molecules are desorbed.
5.
The product diffuse away from the surface of the catalyst.
Related Topics