Surface Chemistry - Theories of Catalysis | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Theories of Catalysis
Theories of Catalysis
For a chemical reaction to occur, the reactants are to be activated to
form the activated complex. The energy required for the reactants to reach the
activated complex is called the activation energy. The activation energy can be
decreased by increasing the reaction temperature. In the presence of a
catalyst, the reactants are activated at reduced temperatures in otherwords,
the activation energy is lowered. The catalyst adsorbs the reactants activates
them by weakening the bonds and allows them to react to form the products.
As activation energy is lowered in presence of a catalyst, more
molecules take part in the reaction and hence the rate of the reaction
increases.
The action of catalysis in chemical reactions is explained mainly by two
important theories. They are
(i) the intermediate compound formation theory
(ii) the adsorption theory.
1. The intermediate compound formation theory
A catalyst acts by providing a new path with low energy of activation.
In homogeneous catalysed reactions a catalyst may combine with one or more
reactant to form an intermediate which reacts with other reactant or decompose
to give products and the catalyst is regenerated.
Consider the reactions:
A+B → AB (1)
A+C → AC (intermediate) (2)
C is the catalyst
AC+B → AB+C (3)
Activation energies for the reactions (2) and (3) are lowered compared
to that of (1). Hence the formation and decomposition of the intermediate accelerate
the rate of the reaction.
Example 1
The mechanism of Fridel crafts reaction is given below
C6 H6 + CH3Cl Anhydrous AlCl3→ C6
H5 CH3 +HCl

The action of catalyst is explained as follows
CH3 Cl+AlCl3 → [CH3 ]+[AlCl4 ]-
It is an intermediate.
C6 H6 +[CH3+][AlCl4
]- → C6
H5CH3 +AlCl3 +HCl
Example 2
Thermal decomposition of KClO3 in presence of MnO2
proceeds as follows.
Steps in the reaction 2KClO 3 → 2KCl+3O2 can be given as
2KClO3 +6MnO2 → 6MnO3 +2KCl
It is an intermediate.
6MnO3 → 6MnO2
+3O2
Example 3:
Formation of water due to the reaction of H2 and O2
in the presence of Cu proceeds as follows. Steps in the reaction H2+½O2
→ H2O
can be given as
2Cu+ 1/2 O 2 → Cu 2O
It is an intermediate.
Cu2 O+H 2 → H2O + 2Cu
Example 4:
Oxidation of HCl by air in presence of CuCl2 proceeds as
follows. Steps in the reaction 4HCl + O2 →2H2O
+ 2Cl2 can be given as
2CuCl 2 → Cl2
+Cu2 Cl2
2Cu2 Cl2 +O2 → 2Cu2OCl2
It is an intermediate.
2Cu2 OCl2 +4HCl → 2H2 O + 4CuCl2
This theory describes
(i) the specificity of a catalyst and
(ii) the increase in the rate of the reaction with increase in the
concentration of a catalyst.
Limitations
(i) The intermediate compound theory fails to
explain the action of catalytic poison and activators (promoters).
(ii) This theory is unable to explain the mechanism of heterogeneous
catalysed reactions.
2. Adsorption theory
Langmuir explained the action of catalyst in heterogeneous catalysed
reactions based on adsorption. The reactant molecules are adsorbed on the
catalyst surfaces, so this can also be called as contact catalysis.
According to this theory, the reactants are adsorbed on the catalyst
surface to form an activated complex which subsequently decomposes and gives
the product.
The various steps involved in a heterogeneous catalysed reaction are
given as follows:
1. Reactant molecules diffuse from bulk to the catalyst surface.
2. The reactant molecules are adsorbed on the surface of the catalyst.
3. The adsorbed reactant molecules are activated and form activated complex
which is decomposed to form the products.
4. The product molecules are desorbed.
5. The product diffuse away from the surface of the catalyst.
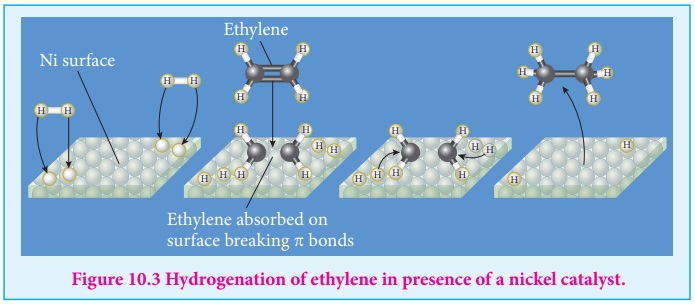
Active centres
The surface of a catalyst is not smooth. It bears steps, cracks and
corners. Hence the atoms on such locations of the surface are co-ordinatively
unsaturated. So, they have much residual force of attraction. Such sites are
called active centres. So, the surface carries high surface free energy.
The presence of such active centres increases the rate of reaction by
adsorbing and activating the reactants.
The adsorption theory explains the following
i. Increase in the surface area of metals and metal oxides by reducing
the particle size increases acting of the catalyst and hence the rate of the
reaction.
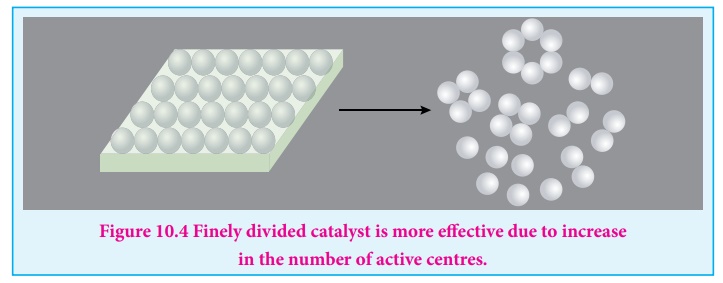
Figure 10.4 Finely divided catalyst is more effective due to
increase in the number of active centres.
ii. The action of catalytic poison occurs when the poison blocks the
active centres of the catalyst.
iii. A promoter or activator increases the number of active centres on
the surfaces.
Related Topics