Surface Chemistry - Properties of Colloids | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Properties of Colloids
Properties of Colloids
1) Colour
2) Size
3) Colloidal solutions are heterogeneous in nature having two distinct phases
4) Filtrability
5) Non-Setting nature
6) Concentration and density
7) Diffusability
8) Colligative properties
9) Shape of colloidal particles
10) Optical property
11) Kinetic property
12) Electrical property
13. Coagulation or precipitation
14. Protective action
1) Colour:
The colour of a sol is not always the same as the colour of the
substance in the bulk. For example bluish tinge is given by diluted milk in
reflected light and reddish tinge in transmitted light.
Colour of the sol, generally depends on the following factors.
• Method of preparation
• Wavelength of source of light.
• Size and shape of colloidal particle
• whether the observer views the reflected light or transmitted light.
2) Size:
The size of colloidal particles ranges from 1nm (10-9m) to
1000 nm (10-6m) diameter.
3) Colloidal solutions are heterogeneous in nature having two distinct phases.
Though experiments like dialysis, ultrafiltration and
ultracentrifuging clearly show the heterogeneous nature in the recent times
colloidal solution are considered as border line cases.
4) Filtrability:
As the size of pores in ordinary filter paper are large the colloidal
particles easily pass through the ordinary filter papers.
5) Non-Setting nature
Colloidal solutions are quite stable i.e. they are not affected by
gravity.
6) Concentration and density
When the colloidal solution is dilute, it is stable. When the volume of
medium is decreased coagulation occurs. Generally, density of sol decreases
with decrease in the concentration.
7) Diffusability
Unlike true solution, colloids diffuse less readily through membranes.
8) Colligative properties
The colloidal solutions show colligative properties i.e. elevation of
boiling point, depression in freezing point and osmotic pressure. Measurements
of osmotic pressure is used to find molecular weight of colloidal particle.
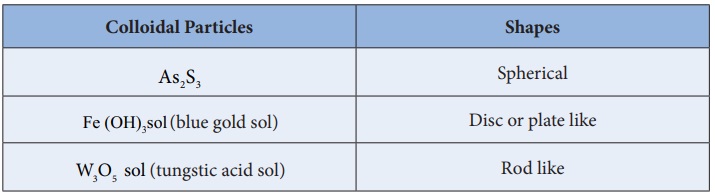
9) Shape of colloidal particles
It is very interesting to know the various shapes of colloidal
particles. Here are some examples
10) Optical property
Colloids have optical property. When a homogeneous solution is seen in
the direction of light, it appears clear but it appears dark, in a
perpendicular direction.
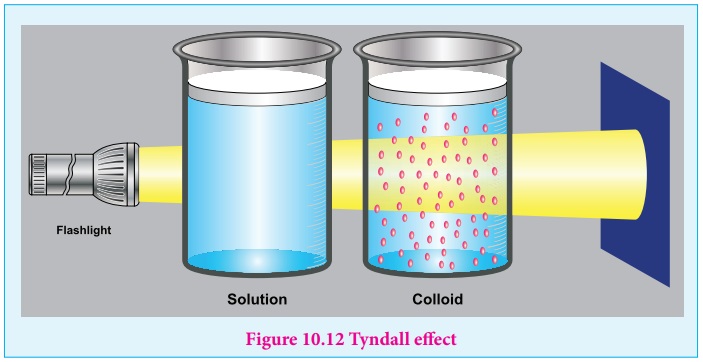
But when light passes through colloidal solution, it is scattered in all
directions. This effect was first observed by Faraday, but investigations are
made by Tyndall in detail, hence called as Tyndall effect.
The colloidal particles absorb a portion of light and the remaining
portion is scattered from the surface of the colloid. Hence the path of light
is made clear.
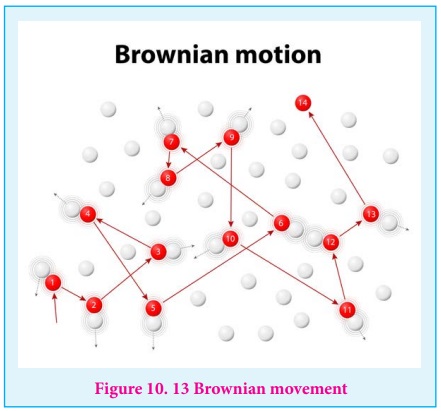
11) Kinetic property
Robert Brown observed that when the pollen grains suspended in water
were viewed through ultra microscope, they showed a random, zigzag ceaseless
motion.
This is called Brownian movement of colloidal particles.
This can be explained as follows
The colloidal sol particles are continuously bombarded with the
molecules of the dispersion medium and hence they follow a zigzag, random,
continuous movement.
Brownian movement enables us,
1. to calculate Avogadro number.
2. to confirm kinetic theory which considers the ceaseless rapid
movement of molecules that increases with increase in temperature.
3. to understand the stability of colloids: As the particles in
continuous rapid movement they do not come close and
hence not get condensed. That is Brownian movement does not allow the
particles to be acted on by force of gravity.
12) Electrical property
(1) Helmholtz double layer
The surface of colloidal particle adsorbs one type of ion due to preferential
adsorption. This layer attracts the oppositely charged ions in the medium and hence
at the boundary separating the two electrical double layers are setup. This is called
as Helmholtz electrical double layer.
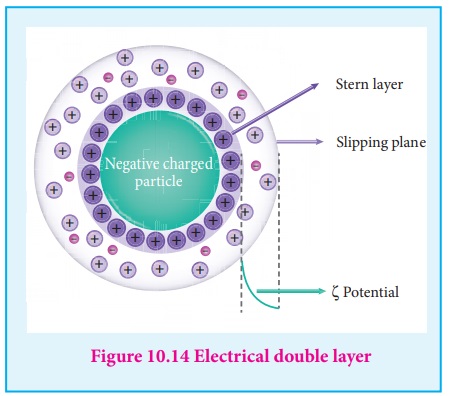
As the particles nearby are having similar charges, they cannot come
close and condense.
Hence this helps to explain the stability of a colloid.
(ii) Electrophoresis:
When electric potential is applied across two platinum electrodes dipped
in a hydrophilic sol, the dispersed particles move toward one or other
electrode.
This migration of sol particles under the influence of electric field is
called electrophoresis or cataphoresis. If the sol particles migrate to the
cathode, then they posses positive (+) charges, and if the sol particles
migrate to the anode then they have negative charges(-).
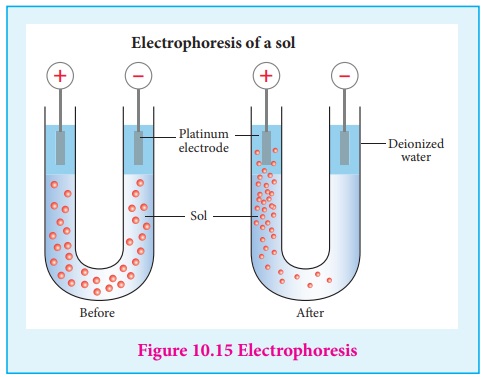
Thus from the direction of migration of sol particles we can determine the charge of the sol particles. Hence electrophoresis is used for detection of presence of charges on the sol particles.
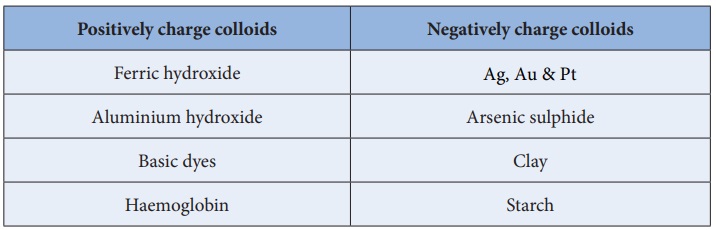
Few examples of charges of sols detected by electrophoresis are
given below:
(iii) Electro osmosis
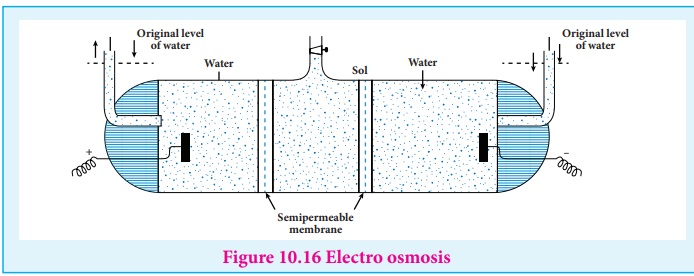
A sol is electrically neutral. Hence the medium carries an equal but
opposite charge to that of dispersed particles. When sol particles are
prevented from moving, under the influence of electric field the medium moves
in a direction opposite to that of the sol particles. This movement of
dispersion medium under the influence of electric potential is called electro
osmosis.
13. Coagulation or precipitation
The flocculation and settling down of the sol particles is called
coagulation.
Various method of coagulation are given below:
(i) Addition of electrolytes
(ii) Electrophoresis
(iii) Mixing oppositively charged sols.
(iv) Boiling
(i) Addition of electrolytes
A negative ion causes the precipitation of positively charged sol and
vice versa.
When the valency of ion is high, the precipitation power is increased.
For example, the precipitation power of some cations and anions varies in the
following order
Al3+ >Ba 2+ >Na+ , Similarly [Fe(CN)6
]3− > SO42− > Cl-
The precipitation power of electrolyte is determined by finding the
minimum concentration (millimoles/lit) required to cause precipitation of a sol
in 2hours. This value is called flocculation value. The smaller the
flocculation value greater will be precipitation.
(ii) Electrophoresis:
In the electrophoresis, charged particles migrate to the electrode of
opposite sign. It is due to neutralization of the charge of the colloids. The
particles are discharged and so they get precipitated.
(iii) By mixing two oppositively charged sols
When colloidal sols with opposite charges are mixed mutual coagulation
takes place. It is due to migration of ions from the surface of the particles.
(iv) By boiling
When boiled due to increased collisions, the sol particles combine and
settle down.
14. Protective action
Generally, lyophobic sols are precipitated readily even with small
amount of electrolytes. But they are stabilised by addition of a small amount
of lyophillic colloid.
A small amount of gelatine sol is added to gold sol to protect the gold
sol.
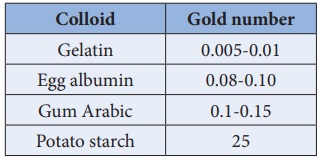
Zsigmondy introduced the term ‘gold number’ as a measure of protecting
power of a colloid. Gold number is defined as the number of milligrams of
hydrophilic colloid that will just prevent the precipitation of 10ml of gold
sol on the addition of 1ml of 10% NaCl solution. Smaller the gold number
greater the protective power.
Related Topics