Surface Chemistry - Adsorption isotherms and isobars | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Adsorption isotherms and isobars
Adsorption isotherms and isobars.
Adsorption isotherms represents the variation of adsorption at constant
temperature.
When amount of adsorption is plotted versus temperature at constant
pressure it is called adsorption isobar.
Adsorption isobars of physisorption and chemisorption are different as
represented in the graphs.
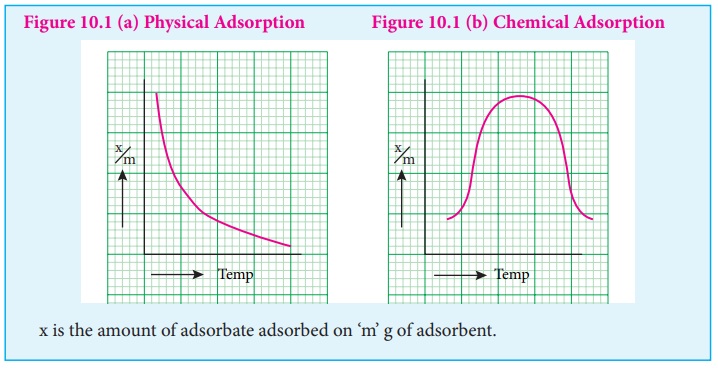
In physical adsorption, x/m decreases with increase in Temprature, But
in chemical adsorption, x/m increases with rise in temperature and then decreases.
The increase illustrates the requirement of activation of the surface for
adsorption is due to fact that formation of activated complex requires certain
energy.
The decrease at high temperature is due to desorption, as the kinetic
energy of the adsorbate increases.
Adsorption isotherms
Adsorption isotherm can be studied quantitatively. A plot between the
amount of adsorbate adsorbed and pressure (or concentration of adsorbate) at
constant temperature is called adsorption isotherms.
In order to explain these isotherms various equations were suggested as
follows:
(i) Freundlich adsorption isotherm.
According to Freundlich,
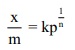
where x is the amount of adsorbate, adsorbed on ‘m’ gm of adsorbent at a
pressure of p. K and n are constant introduced by freundlich.
Value n is always less than unity.
This equation is applicable for adsorption of gases on solid surfaces.
The same equation becomes x/m = Kc1/n , when used for adsorption in
solutions with c as concentration.
This equation quantitively predicts the effect of pressure(or
concentration) on the adsorption of gases(or adsorbates) at constant
temperature.
Taking log on both sides of equation x/m =Kp1/n
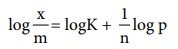
log x/m = logK + 1/n log p
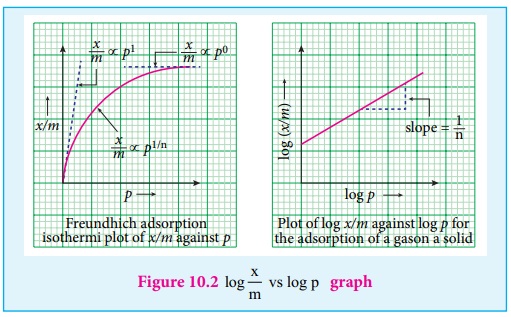
Hence the intercept represents the value of log k and the slope gives 1/
n.
This equation explains the increase of x/m with increase in pressure.
But experimental values show the deviation at low pressure.
Limitations
This equation is purely empirical and valid over a limited pressure
range.
The values of constants k and n also found vary with temperature. No
theoretical explanations were given.
Related Topics