Surface Chemistry - Purification of colloids | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Purification of colloids
Purification of colloids
The colloidal solutions due to their different methods of preparation
may contain impurities. If they are not removed, they may destablise and
precipitate the colloidal solution. This is called coagulation. Hence the
impurities mainly electrolytes should be removed to increase the stabilisation
of colloid. Purification of colloidal solution can be done by the following
methods.
(i) Dialysis
(ii)
Electrodialysis
(iii)
Ultrafilteration.
(i) Dialysis
In 1861, T. Graham separated the electrolyte from a colloid using a
semipermeable membrane (dialyser). In this method, the colloidal solution is
taken in a bag made up of semipermeable membrane. It is suspended in a trough
of flowing water, the electrolytes diffuse out of the membrane and they are
carried away by water.
Do you Know? Kidney malfunction results in the
building up of electrolyte concentration
within the blood to toxic levels.
In the Dialysis, recycling of patient’s blood is done through
considerable length of seimpermeable tube in an isotonic saline solution.
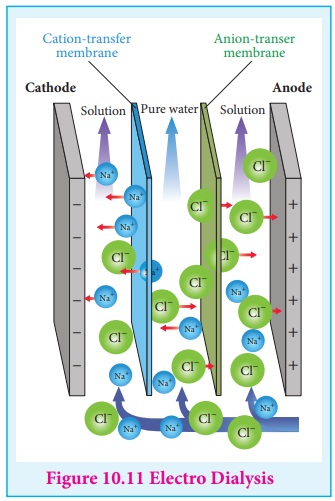
(ii) Electrodialysis
The presence of electric field increases the speed of removal of
electrolytes from colloidal solution. The colloidal solution containing an
electrolyte as impurity is placed between two dialysing membranes enclosed into
two compartments filled with water. When current is passed, the impurities pass
into water compartment and get removed periodically. This process is faster
than dialysis, as the rate of diffusion of electrolytes is increased by the
application of electricity.
(iii) Ultrafiltration
The pores of ordinary filter papers permit the passage of colloidal
solutions. In ultra filtrations, the membranes are made by using collodion
cellophane or visiking. When a colloidal solution is filtered using such a
filter, colloidal particles are separated on the filter and the impurities are
removed as washings. This process is quickened by application of pressure. The
separation of sol particles from electrolyte by filteration through an
ultrafilter is called ultrafiltration. Collodion is 4% solution of
nitrocellulose in a mixture of alcohol and water.
Related Topics