Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Applications of adsorption
Applications of adsorption
Though we have innumerable applications for adsorption, we consider few
of them
1. Gas masks: During world war I charcoal gas mask
was employed by both the British and American. Activated charcoal was found to
be one of the best adsorbents.
2. To create high vacuum in vessels, Tail and Dewar
used activated charcoal.For dehydration and also purification of gases like CO2,
N2, Cl2, O2 and He, alumina and silica are
employed. In the blast furnace silica gel is also used for drying air.
3. One of the highly important use of adsorption is
the softening of hardwater. Permutit is employed for this process which adsorbs
Ca2+ and Mg2+ ions in its surface, there is an ion
exchange as shown below it occurs on the surface.
Na2 Al2 Si4 O12 + CaCl 2
→ CaAl2
Si4 O12 + 2NaCl
Exhausted permutit is regenerated by adding a solution of common salt.
CaAl2 Si4O12 + 2NaCl → Na2
Al2 Si4 O12 + CaCl2
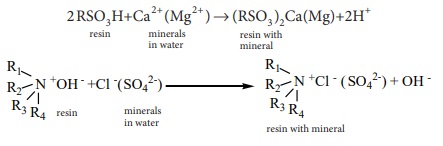
4. Ion exchange resins
Ion exchange resins are working only based on the process of adsorption.
Ion exchange resins are used to demineralise water. This process is carried out
by passing water through two columns of cation and anion exchange resins.
2RSO3 H + Ca2+ (Mg2+ ) →(RSO3)2
Ca(Mg) + 2H+
5. Petroleum refining and refining of vegetable oil
Fuller’s earth and silica gel are used for refining process.
6. Decolourisation of Sugar:
Sugar prepared from molasses is decolourised to remove coloured
impurities by adding animal charcoal which acts as decolourising material.
7. Chromatography
The chromatographic technique is applied for separation of components in
a mixture. It is mainly based on adsorption of components on the surface of
adsorbents. This method is very effective and used for identification,
detection and estimation of many substances even if they are contained in micro
quantities.
8. Catalysed reaction
Catalysis is an important branch of surface chemistry which is based on
the phenomenon of adsorption of materials on the catalyst surface.
Examples:
In the Haber’s process, ammonia is manufactured from N2 and H2
as shown by the following reactions.
N2 + 3H2 → 2NH3
In this process, Fe is the catalyst and Mo is a promoter. The surface of
the Fe catalyses the reaction.
In the hydrogenation of oils to obtain vanaspathi, Nickel is used as a
catalyst. Nickel surface catalyses the reaction.
vegetable oil + H2 →Ni catalyst473K→ vanaspathi
9. Qualitative analysis
When blue litmus solution is added to Al3+ ion, a red
coloration is seen due to the acidic nature of the solution. Addition of
ammonium hydroxide to it gives a blue lake. This is due to the adsorption of
blue colour litmus compound on the surface of Al (OH)3 Which is
formed during the addition of NH4OH
10. Medicine:
Drugs cure diseases by adsorption on body tissues.
11. Concentration of Ores of metals
Sulphides ores are concentrated by a process called froth flotation in
which light ore particles are wetted by pine oil.
12. Mordants and Dyes
Most of the dyes are adsorbed on the surface of the fabrics. Mordants
are the substances used for fixing dyes onto the fabric.
13. Adsorption indicators
In the precipitation titrations, the end point is indicated by an
external indicator which changes its colour after getting adsorbed on
precipitate. It is used to indicate the end point of the titration.
Related Topics