Chapter: Biochemistry: Nucleic Acid Biotechnology
What are plasmids?
What are plasmids?
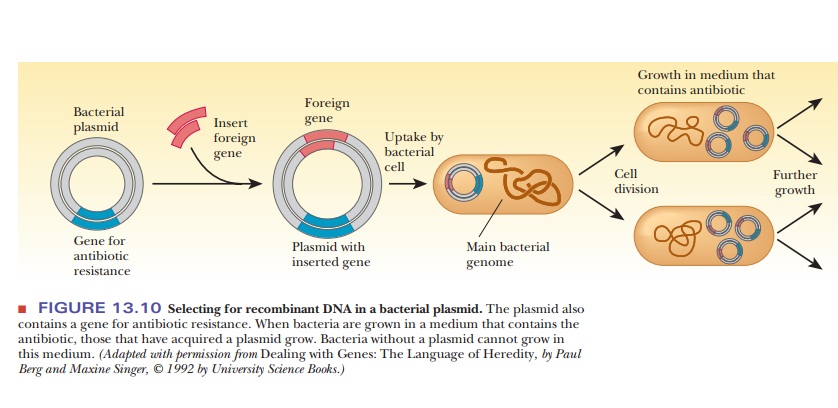
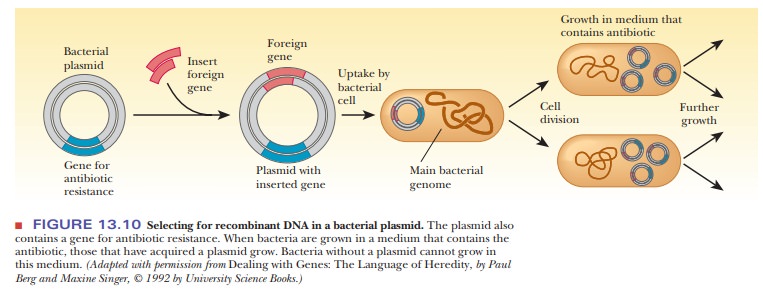
The other principal vector is a plasmid-bacterial DNA that is not part of the main circular DNA chromosome of the bacterium.
This DNA, which usually exists
as a closed circle, replicates independently of the main bacterial genome and
can be transferred from one strain of a bacterial species to another by cell-to-cell
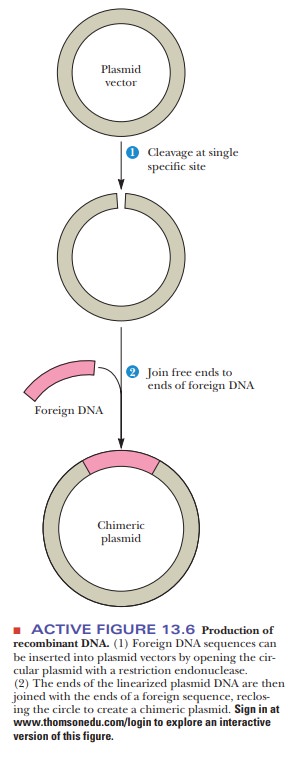
contact. The foreign gene can be inserted into the plasmid by the successive
actions of restriction endonucleases and DNA ligase, as was seen in Figure
13.6. When the plasmid is taken up by a bacterium, the DNA insert goes along
for the ride (Figure 13.10). The bacteria that contain the DNA insert can then
be grown in fermentation tanks under conditions that allow them to divide
rapidly, amplifying the inserted gene many thousandfold.
While
the theory of cloning DNA into a plasmid is straightforward, there are several
considerations for a successful experiment. When bacteria take up a plasmid, we
say they have been transformed.
Transformation is the process whereby new DNA is incorporated into a host.
Bacteria are encouraged to take up foreign DNA by a couple of methods. One is
to heat-shock the bacteria at 42°C, followed by placing them on ice. Another is
to place them in an electric field, a technique called electroporation.
How are we to know which of the bacteria have taken up the plasmid? Because bacteria divide quickly, we would not want all the bacteria to grow-rather, only the ones that have the plasmid. This process is called selection. Each plasmid chosen for cloning must have some type of selectable marker that lets us know that the growing bacterial colonies contain the plasmid. These markers are usually genes that confer resistance to antibiotics. After transformation, the bacteria are plated on a medium containing the antibiotic to which the plas-mid carries resistance.
In that way, only the bacteria that took up the plasmids will grow
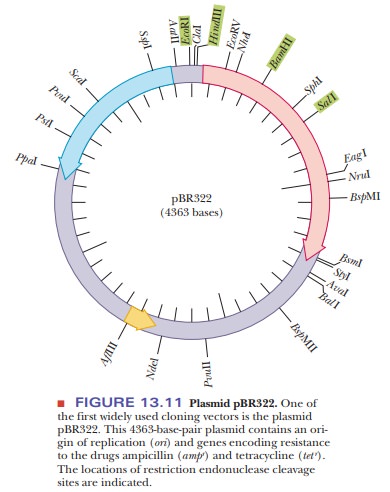
(Figure 13.10). One of the first plasmids used for cloning is pBR322 (Figure
13.11). This simple plasmid was created from a naturally occurring one found in
E. coli. Like all plasmids, it has an
origin of replication, so it can rep-licate independently of the rest of the
genome. It has genes that confer resis-tance to two antibiotics, tetracycline
and ampicillin. The genes are indicated tet
rand ampr. The
pBR322 plasmid has several restriction enzyme sites. Thenumber and location of
restriction sites is very important to a cloning experi-ment. The foreign DNA
must be inserted at unique restriction sites so that the use of restriction
enzymes opens up the plasmid at only one point. Also, if the restriction site
chosen is inside one of the selection markers, the resistance to the antibiotic
is lost upon insertion of the foreign DNA. This was, in fact, the original way
selection was done with plasmids. Foreign DNA was inserted using restriction
sites in the tetr gene. Selection was achieved
by noting the loss of the ability of bacteria to grow on a medium containing
tetracycline.
One of
the early stumbling blocks to cloning was finding the right plasmid, one that
had restriction sites that matched those enzymes needed to cut out the foreign
DNA. As the technology to design plasmids improved, they were created with
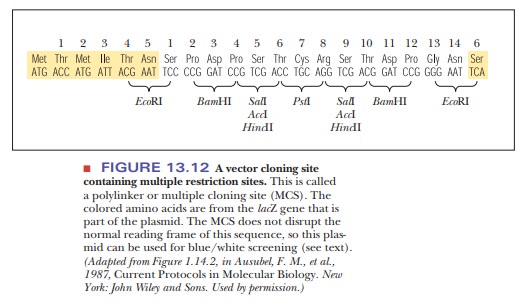
regions that had many different restriction sites in a small space. This region
was called a multiple cloning site (MCS)
or a polylinker (Figure 13.12). A
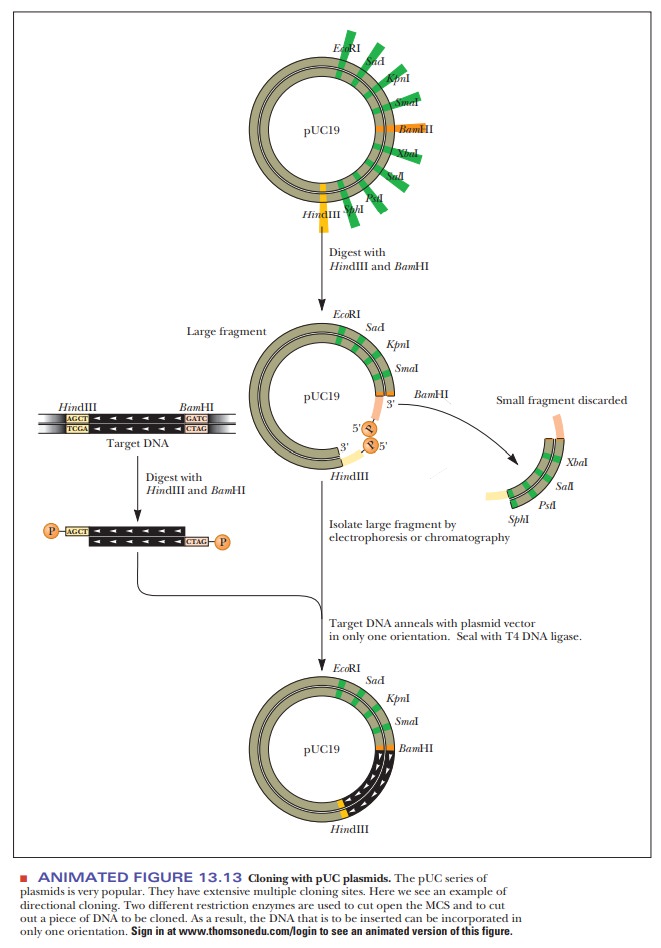
popular cloning vector series is based on the pUC plasmids (Figure 13.13). The
acronym pUC stands for universal cloning plasmid. Each of
these cloning vectors has an extensive MCS, which helps solve another problem
with
Depending on what is to be done with the
cloned DNA, controlling its orientation in the vector may be important. If only
one restriction enzyme is used, such as BamH1,
then the foreign DNA can enter the plasmid in either of two directions.
However, if the foreign DNA is cut out of its source at one end with BamH1 and at the other end with HindIII, and if these same two
restriction enzymes are used to open up the plasmid, then the ends match in
only one direction (Figure 13.13).
The use of the pUC plasmids also aids in the selection procedure. The older plasmids that were based on pBR322 had the shortcoming that the foreign DNA was inserted into the tetracycline resistance gene. This meant that the only way to spot bacteria that had taken up a plasmid that had also taken up the insert was that the bacteria would not grow on a medium containing tet-racycline. This lack of growth by the successful clone made it challenging to go back and find the proper bacterial colonies. The pUC plasmids, however, have a characteristic that alleviates this procedure-they contain the lacZ gene, which is the basis for a selection technique called blue/white screening. The lacZ gene codes for theα-subunit of the enzymeβ-galactosidase, which is used to cleave disaccharides, such as lactose.
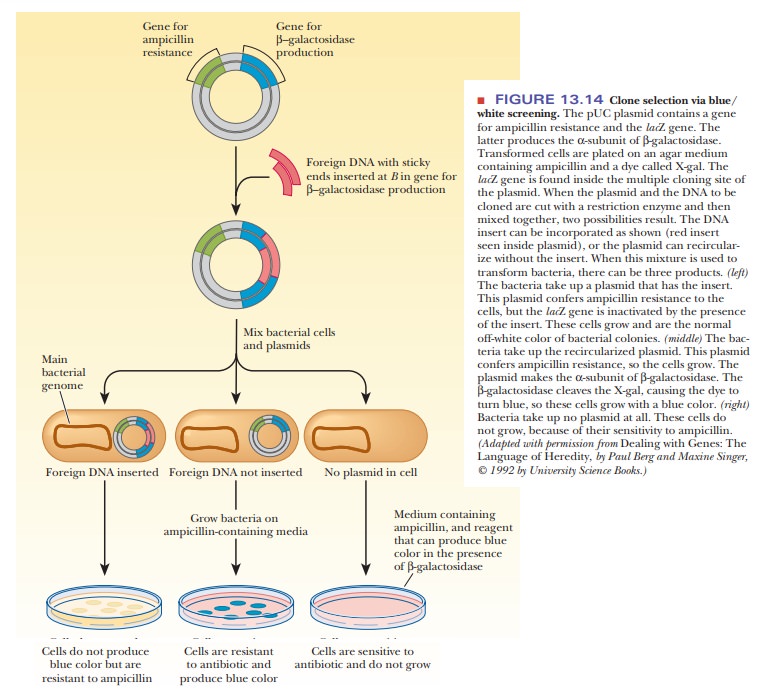
The MCS is located inside the lacZ gene, so that, when foreign DNA is
inserted, it inactivates the gene. Figure 13.14 shows how this characteristic
is useful. The pUC plasmid is cut open in its MCS by restriction enzymes, and
the foreign DNA (red) is cut out of its source with the same enzymes. These are
then combined and joined together with DNA ligase to yield two products in the
ligation reaction. The desired product is the plasmid that now contains the
foreign DNA. The other is a plasmid that has reclosed upon itself without the
inserted DNA. This type is much less common when two different restriction
enzymes are used to open the plasmid, but it still occurs infrequently. When
this mixture is used to trans-form the bacteria, there are three possible
products: (1) bacteria that took up the plasmid with the insert, (2) bacteria
that took up the plasmid without the insert, and (3) bacteria that took up no
plasmid at all. The mixture of bacteria from the transformation is plated on a
medium containing ampicillin and a dye, such as X-gal. β-Galactosidase hydrolyzes a bond in the X-gal
molecule, turning it blue. The bacteria to be transformed are mutants that make
a defec-tive version of the β-galactosidase that lacks the α-subunit. If the bacteria
take up no plasmid at all, they lack the ampicillin-resistance gene and do not
grow. If the bacteria take up a plasmid lacking the insert, they have a
functional lacZ gene and produce the α-subunit of β-galactosidase. These
colonies produce active β-galactosidase, which cleaves the dye, X-gal.
These colonies grow with a blue color. If the bacteria take up a plasmid that
contains the insert, the lacZ gene is
inactivated. These colonies are the off-white color of normal bacterial
colonies on agar.
Related Topics