Chapter: Biochemistry: Nucleic Acid Biotechnology
Genetic Engineering
Genetic Engineering
The
previous sections dealt with how DNA of interest could be inserted into a
vector and amplified by cloning. One of the most important purposes for doing
this is to be able to produce the gene product in larger quantities than could
be acquired by other means. When an organism is intentionally changed at the
molecular level so that it exhibits different traits, we say it has been geneticallyengineered.
In a sense, genetic engineering on an organismal level has been around since humans first started to use selective breeding on plants and animals. This pro-cedure did not deal directly with the molecular nature of genetic material, nor was the appearance of traits under human control. Breeders had to cope with changes that arose spontaneously, and the only choice was whether to breed for a trait or to let it die out. An understanding of the molecular nature of heredity and the ability to manipulate those molecules in the laboratory have, of course, added to our ability to control the appearance of these traits.
What is the purpose of genetic engineering?
The
practice of selective alteration of organisms for both agricultural and medical
purposes has profited greatly from recombinant DNA methods. Genetic engineering
of crop plants is an active field of research. Genes for increased yields,
frost resistance, and resistance to pests are introduced into commercially
important plants such as strawberries, tomatoes, and corn. Similarly, animals
of commercial importance-mostlymammals,butalsoincludingfish-arealsogeneticallyaltered.
Some variations introduced in animals have medical implications. Mice with
altered genetic makeup are used in the research laboratory. In another
medically related field, researchers working with insect-borne diseases, such
as malaria, are trying to engineer strains of insects, such as the mosquito, Anopheles gambiae, that can no longer
transmit the infection to humans (Figure 13.15). In all cases, the focus of the
research is to introduce traits that can
be inherited by the descendants of the treated organisms. In the treatment
of human genetic disease, however, the aim is not to produce heritable changes.
Serious ethical questions arise with the manipulation of human genetics;
consequently, the focus of research has been on forms of gene therapy in which cells of specific tissues in a living person
are altered in a way that alleviates the effects of the disease. Examples of
diseases that may someday be treated in this way include cystic fibrosis,
hemophilia, Duchenne muscular dystrophy, and severe combined immune deficiency
(SCID). The last of these is also known as “bubble-boy syndrome,” because those
who have it must live in isolation (in a large “bubble”) to avoid infection.

DNA Recombination Occurs in Nature
When
recombinant DNA technology was in its early stages in the 1970s, considerable
concern arose both about safety and about ethical questions. Some of the
ethical questions are still matters of concern. One that has definitely been
laid to rest is the question of whether the process of cutting and splicing DNA
is an unnatural process. Indeed, DNA recombination is a common part of the
crossing over of chromosomes. There are many, varied reasons for in vivo
recombination of DNA, two of which are the maintenance of genetic diversity and
the repair of damaged DNA.
Until
recently, heritable changes in organisms were solely those that arose from
mutations. Researchers in the field took advantage of both spontaneous
mutations and those produced by exposure of organisms to radioactive materi-als
and other substances known to induce mutations. Selective breeding was then
used to increase the population of desired mutants. It was not possible to
produce “custom-tailored” changes in genes.
Since
the advent of recombinant DNA technology, it is possible (within limits) to
change specific genes, and even to change specific DNA sequences within those
genes, to alter the inherited characteristics of organisms. Bacteria can be
altered to produce large amounts of medically and economically important
proteins. Animals can be manipulated to cure, or to alleviate the symptoms of,
their genetic diseases, and agriculturally important plants can be made to
produce greater crop yields or be given increased resistance to pests. The
following Biochemical Connections box gives some examples of agricultural
applications of genetic engineering.
Bacteria as “Protein Factories”
We can
use the reproductive power of bacteria to express large quantities of a mammalian
protein of interest; however, the process is often more complicated than it
might seem because most mammalian proteins are heavily processed after their
initial transcription and translation. Because bacteria have little
posttranslational modification of their proteins, they lack the enzymes
necessary for this processing.
How can human proteins be made by bacteria?
An
application of genetic engineering that is of considerable practical importance
is the production of human insulin by E.
coli. This was one of the first human proteins produced through genetic
engineering, and its production eliminated the problems related to harvesting
insulin from large numbers of laboratory animals and giving humans a peptide
from another species. The process is far from straightforward, however. A
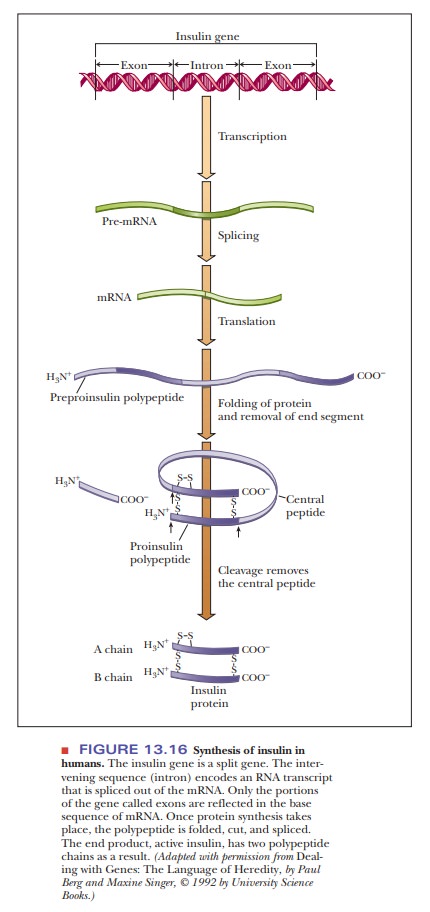
significant problem is that the insulin gene is split. It contains an intron, a DNA sequence that codes for
RNA that is eventually deleted in the processing of the mRNA that directs the
synthesis of the protein. Only the RNA transcribed from DNA sequences called exons appears in mature mRNA (Figure
13.16). Bacteria do not have the cellular apparatus for splicing introns out of
RNA transcripts to give functional mRNA. One might think that the problem could
be solved by using cDNA obtained from the mRNA for insulin in a reaction
catalyzed by reverse transcriptase. The problem here is that the polypeptide
encoded by this mRNA contains an end peptide and a central peptide, which is to
be removed from it by further processing in insulin-producing cells to yield
two polypeptide chains, designated A and B (Figure 13.16).
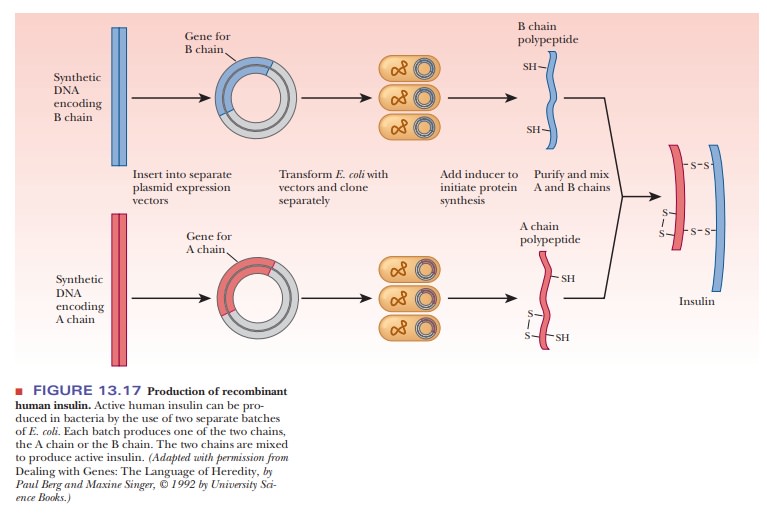
The
approach to this problem is to use two synthetic DNAs, one encoding the A chain
of insulin and the other encoding the B chain. These synthetic DNAs are produced
in the laboratory using methods that were developed by synthetic organic
chemists. Each DNA is inserted into a separate plasmid vector (Figure 13.17).
The vectors are taken up by two different populations of E. coli. The two groups are then cloned separately; each group of
bacteria produces one of the two polypeptide chains of insulin. The A and B
chains are extracted and mixed, finally producing functional human insulin.
Protein Expression Vectors
The
plasmid vectors pBR322 and pUC are referred to as cloning vectors. They are used to insert the foreign DNA and to
amplify it. However, if the goal is to produce the protein product from the
foreign DNA, they are not suitable. An expression
vector is needed.
What is an expression vector?
An expression vector has many of the same attributes as a cloning vector, such as the origin of replication, a multiple cloning site, and at least one selectable marker. In addition, it must be able to be transcribed by the genetic machinery of the bacteria into which it is transformed. This means that it must have a promoter for RNA polymerase, and the RNA transcribed must have a ribosomal binding site so that it can be translated. It must also have a transcription termination sequence; otherwise, the entire plasmid is transcribed instead of just the inserted gene. Figure 13.18 shows a schematic of an expression vector. Upstream of the site where the foreign DNA is inserted is the transcription promoter. Often this is the promoter for a viral RNA polymerase called T7polymerase. There is also a T7 terminator at the other end of the MCS. After theinsert is successfully ligated, the plasmid is transformed into an expression strain of bacteria, such as E. coli JM109 DE3. What makes this strain unique is that it has a gene that produces T7 RNA polymerase, but the gene is under the control of the lac operon. Once the bacteria are growing well with the plasmid, the cells are given a lactose analogue, IPTG (isopropylthiogalactoside). This stimulates the lac operon in the bacteria, which then produce T7 RNA polymerase, which then binds to the plasmid T7 promoter and transcribes the gene. The bacterial cells then translate the mRNA into protein. This selective control of the expression is important because many foreign proteins are toxic to the cells; expression must be timed carefully.
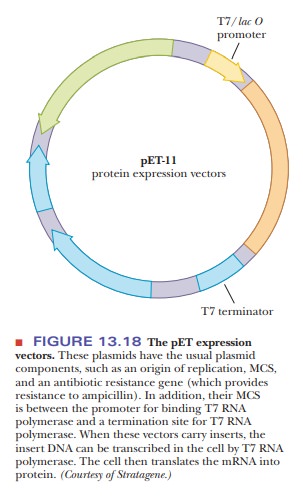
The plasmid shown in Figure 13.18
also has the lacI gene, although it
is transcribed in the opposite direction. This produces the repressor for the lac operon to help make sure that none
of the foreign proteins are transcribed unless the system is induced by IPTG.
The following Biochemical Connections box gives an example of how protein
expression can be linked to a novel purification scheme.
Genetic Engineering in Eukaryotes
When the target organism for genetic engineering is an animal or a plant, one must consider that these are multicellular organisms with multiple kinds of tissues. In bacteria, altering the genetic makeup of a cell implies a change in the whole single-celled organism. In multicellular organisms, one possibility is to change a gene in a specific tissue, one that contains only one kind of differentiated cell. In other words, the change is somatic, affecting only the body tissues of the altered organism.
In contrast, changes
in germ cells (egg and sperm cells), called germ-line
changes, are passed on to succeeding generations. If germ cells are to be
modified, the change must be made at an early stage in development, before the
germ cells are sequestered from the rest of the organism. Attempts to produce
such changes have succeeded in comparatively few organisms, such as plants,
fruit flies, and some other animals such as mice. Genetic engineering in plants
frequently uses a vector based on a bacterial plasmid from the crown gall
bacterium, Agrobacterium tumefaciens.
Cells of this bacterium bind to wounded plant tissue, allowing plasmids to move
from the bacterial cells into the plant cells. Some of the plasmid DNA inserts
itself into the DNA of the plant cells in the only known natural transfer of
genes from a bacterial plasmid to a eukaryotic genome. Expression of plasmid
genes in the plant gives rise to a tumor called a crown gall. Whole, healthy plants can grow from gall cells, even
though they are not germ cells. (This process, of course, does not take place
in animals.) The plants that grow from the gall cells can produce fertile
seeds, allowing the gene that has been transferred to be continued in a new
strain of the plant. Genes from any desired source can be incorporated into the
A. tumefaciens plasmid and then
transferred to a plant. This method was used to genetically engineer tomato
plants that resist defoliation by caterpillars (Figure 13.19). A gene that
encodes a protein toxic to caterpillars was taken from the bacterium Bacillus thuringensis to bring about
this modification. Work is continuing on other useful modifications of food
crops. Many observers of this whole line of research have raised questions
about both the safety and the ethics of the process. The public became more
aware of the extent to which genetically modified (GM) foods were in
circulation in 2000, when corn that had been modified with the gene from Bacillus thuringensis (Bt corn) showed
up in taco shells. This had been an accident, as the Bt corn had been approved
only for animal feed and not for human consumption, pending studies of
potential allergenic effects. Environmentalists are also concerned about the
effect of GM crops for two reasons. The first concern is the effect on
nontargeted insects, such as the monarch butterfly, which may be particularly
Second is the potential to create a super breed of insect accidentally that is
immune to the effect of the toxin. On the positive side, fields planted with Bt
cotton plants can sometimes use up to 80% less pesticide than fields planted
with ordinary cotton.
Summary
Genetic
engineering is the process of inserting genes of interest into spe-cific
organisms for either a medical or purely scientific benefit.
Gene
therapy is the process of inserting a missing gene into an organism.
Bacteria are often used as the factories to produce a protein from
a cloned gene. This has led to the production of human proteins such as insulin
and erythropoietin.
To produce the protein product of a gene of interest, the gene must
be cloned into an expression vector, usually a plasmid with special features
that allows it to be transcribed and translated in a host cell.
In agriculture, genetic engineering is used to
produce crops that are resis-tant to insects or have long shelf lives.
Related Topics