Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Volatilization Gravimetry: Theory and Practice
Theory and Practice
Whether the analysis is direct or indirect, volatilization gravimetry requires that the products of the decomposition reaction be known. This requirement is rarely a problem for organic compounds for which volatilization is usually accomplished by combustion and the products are gases such as CO2, H2O, and N2. For inorganic compounds, however, the identity of the volatilization products may depend on the temperature at which the decomposition is conducted.
Thermogravimetry
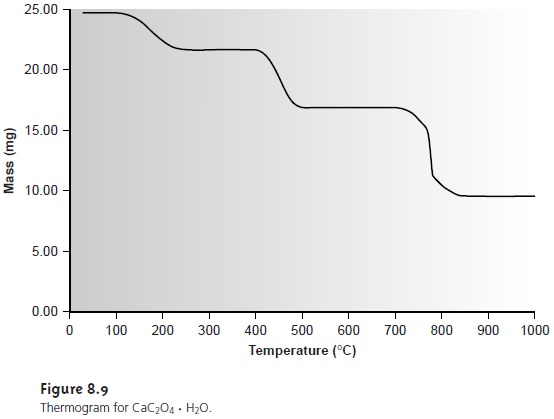
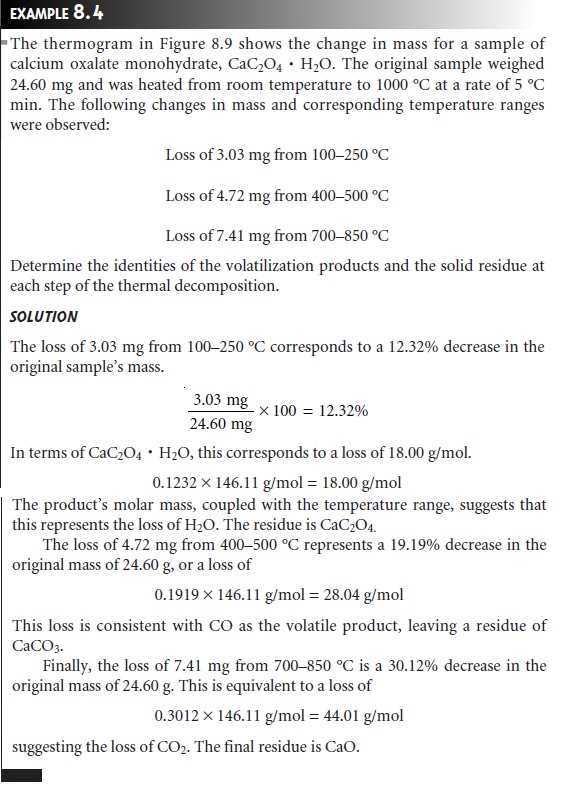
The products of a thermal decomposition can be deduced by monitoring the sample’s mass as a function of applied temperature. (Figure 8.9). The loss of a volatile gas on thermal decomposition is indicated by a step in the thermogram. As shown in Example 8.4, the change in mass at each step in a ther- mogram can be used to identify both the volatilized species and the solid residue.
Once the products
of thermal decomposition have been determined, an analyt- ical procedure
can be developed. For example,
the thermogram in Figure 8.9 shows
that a precipitate of CaC2O4 H2O must be heated
at temperatures above
250 °C, but below
400 °C if it is to be isolated as CaC2O4. Alternatively, by heating the
sam- ple to 1000 °C, the precipitate can be isolated
as CaO. Knowing
the identity of the
volatilization products also makes it possible to design an analytical method
in which one or more of the gases
are trapped. Thus,
a sample of CaC2O4 H2O could be analyzed by heating to 1000 °C and passing
the volatilized gases through a trap
that selectively retains
H2O, CO, or CO2.
Equipment
Depending on the method,
the equipment for volatilization gravime- try may be simple
or complex. In the simplest
experimental design, the weight of a
solid residue is determined following
either thermal decomposition at a fixed tem-
perature or combustion. Thermal decomposition or combustion is accomplished
using a Bunsen or Meker
burner, a laboratory oven or a muffle furnace,
with the volatile products
vented to the atmosphere. The weight of the sample and the solid
residue are determined using an analytical balance.
Constant-temperature decomposition or combustion, followed
by trapping and weighing the
volatilized gases, requires more specialized equipment. Decom- position of the sample is conducted in a closed container, and the volatilized
gases are carried
by a purge-gas stream through
one or more
selective absorbent traps.
In a thermogravimetric analysis, the sample is placed in a small weighing
boat attached to one arm of a specially
designed electromagnetic balance and placed inside an electric
furnace. The temperature of the electric furnace is slowly increased
at a fixed rate of a few degrees
per minute, and the sample’s weight is monitored.
Representative Method
Although each volatilization gravimetric procedure has its own unique characteristics, the following indirect method for the determination of Si in ores and alloys by formation of volatile SiF4 provides an instructive example of a typical procedure.
Related Topics