Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Theory and Practice of Precipitation Gravimetry: Avoiding Impurities
Avoiding Impurities
Precipitation gravimetry is based on a known
stoichiometry between the analyte’s
mass and the mass of a precipitate. It follows, therefore, that the precipitate must be free from impurities. Since precipitation typically
occurs in a solution
rich in dissolved solids, the initial
precipitate is often
impure. Any impu- rities present in the precipitate’s matrix must be removed
before obtaining its weight.
The greatest source of impurities results from chemical
and physical interac- tions occurring at the
precipitate’s surface. A precipitate is generally crystalline, even if only on a microscopic scale, with a well-defined lattice
structure of cations and anions. Those cations
and anions at the surface
of the precipitate carry, respec-
tively, a positive or a negative charge
as a result of their
incomplete coordination
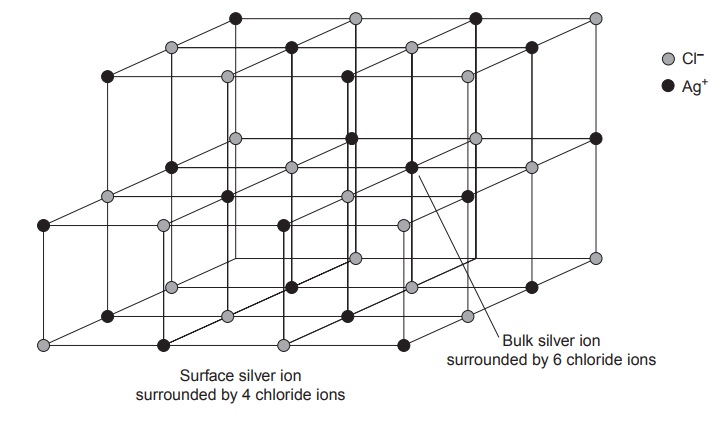
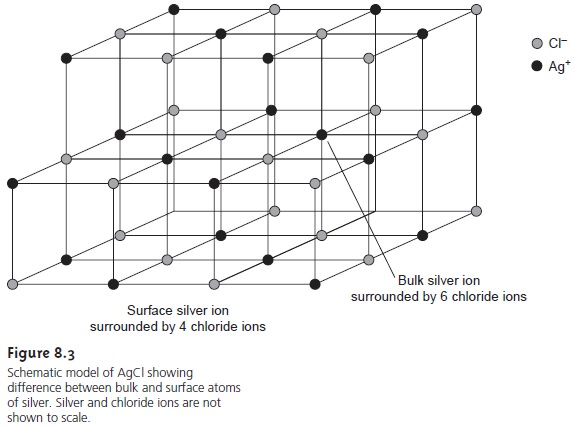
spheres. In a precipitate of AgCl, for example, each Ag+ ion in the bulk of the pre-
cipitate is bound to six Cl– ions. Silver ions at the surface,
however, are bound to no more
than five Cl– ions, and
carry a partial
positive charge (Figure
8.3).
Precipitate particles grow in size because of the electrostatic attraction between
charged ions on the surface
of the precipitate and oppositely charged ions in solu-
tion. Ions common to the precipitate are chemically adsorbed, extending the crystal lattice. Other ions may be physically adsorbed and, unless displaced, are incorpo-
rated into the crystal lattice
as a coprecipitated impurity. Physically adsorbed ions are less
strongly attracted to the surface
and can be displaced by chemically adsorbed ions.
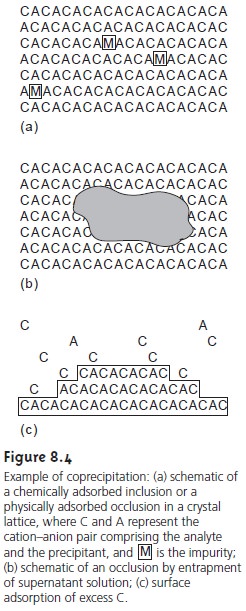
One common type of impurity
is an inclusion. Potential interfering ions whose size and charge are
similar to a lattice ion
may substitute into
the lattice structure by chemical adsorption, provided that the interferent precipitates with the
same crystal structure (Figure
8.4a). The probability of forming an inclusion is greatest when the
interfering ion is present at substantially higher concentrations than the dissolved lattice ion. The presence
of an inclusion does not decrease the amount of analyte
that precipitates, provided
that the precipitant is added in sufficient excess.
Thus, the precipitate’s mass is always
larger than expected.
Inclusions are difficult to remove since
the included material
is chemically part of
the crystal lattice.
The only way to remove
included material is through reprecip- itation. After isolating the precipitate from the supernatant solution, it is dissolved
in a small portion of a suitable
solvent at an elevated temperature. The solution is then cooled to re-form the
precipitate. Since the concentration ratio of
interferent to analyte is lower in the new
solution than in the original supernatant solution, the mass percent of included
material in the precipitate decreases. This process of re-
precipitation is repeated as needed to completely remove the inclusion.
Potential solubility losses of the analyte, however, cannot be ignored. Thus, reprecipitation requires a precipitate of low solubility, and a solvent
for which there
is a significant difference in the precipitate’s solubility as a function
of temperature.
Related Topics