Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Volatilization Gravimetry: Quantitative Applications
Quantitative Applications
Unlike precipitation gravimetry, which is rarely
used as a standard method
of analy- sis, gravimetric
methods based on volatilization reactions continue to play an im- portant role in chemical
analysis. Several important
examples are discussed
in the following sections.
Inorganic Analysis
Determining the inorganic ash
content of organic
materials, such as polymers
and paper, is an example
of a direct volatilization gravimetric analysis. The sample is weighed, placed in an appropriate crucible,
and the organic material is carefully removed by combustion. The crucible containing the residue is then
heated to a constant weight
using either a burner or an oven.
Another example of volatilization gravimetry is the determination of dissolved solids in water and wastewater. In this method a sample of the water is transferred
to a weighed dish and dried to a constant
weight at either
103–105 °C, or at 180 °C.
Samples dried at the lower
temperature retain some occluded water
and lose some carbonate as CO2. The loss of organic material, however, is minimal.
At the higher temperature, the residue
is free from occluded water,
but losses of carbonate are greater. In addition, some chloride,
nitrate, and organic material are lost through
thermal decomposition. The residue remaining after drying at either temperature
can be ignited to constant weight at 500
°C. The loss
in weight on ignition provides an indirect measure of the amount
of volatile solids
in the sample, and the weight of the
remaining residue gives
the amount of fixed solids.
Indirect analyses based on the weight of the residue
remaining after volatiliza- tion are commonly used in determining moisture in a variety of products and in de- termining silica in water,
wastewater, and rocks.
Moisture is determined by drying a preweighed sample with an infrared lamp or in a low-temperature oven. The differ- ence between the original
weight and the weight after
drying equals the mass of water
lost.
The determination of silicon is commonly encountered in metallurgical and mining
laboratories responsible for the analysis of
ores, slags, and alloys. The volatilization gravimetric method, which is appropriate for
samples containing high concentrations of silicon, was described earlier
in Method 8.2.
As a final
example, the determination of carbon in steels and other metal
alloys can be determined by heating the sample. The carbon is converted to CO2, which is
collected in an appropriate absorbent trap, providing a direct
measure of the amount of C in the original
sample.
Organic Analysis
The most important application of volatilization gravimetry to the analysis of organic materials is an elemental analysis. When burned
in a stream of pure O2, many elements, such as carbon and hydrogen,
are released as gaseous
combustion products, such as CO2 and H2O. The combustion products
are passed through preweighed tubes containing appropriate absorbents. The increase
in the mass of these tubes provides a direct indication of the mass percent of carbon and hydrogen in the organic
material.
Alkaline metals and earths
in organic materials can be determined by adding H2SO4 to the sample before combustion. Following combustion, the metal remains behind as a solid
residue of metal
sulfate. Silver, gold,
and platinum can
be deter- mined by burning the
organic sample, leaving
a metallic residue
of Ag, Au,
or Pt. Other metals are determined by adding HNO3 before combustion, leaving
a residue of the metal oxide.
Volatilization gravimetry is also used to determine biomass in water
and waste- water. Biomass
is a water quality index,
providing an indication of the total
mass of organisms contained within a sample
of water. A known volume
of the sample
is passed through a preweighed 0.45-μm
membrane filter or a glass-fiber filter and dried at 105 °C for 24 h. The residue’s mass provides a direct measure
of biomass. If samples are known to contain a substantial amount of dissolved
inorganic solids, the residue
can be ignited at 500 °C for 1 h, thereby removing
all organic materials. The resulting residue is wetted with distilled water to rehydrate
any clay minerals and dried to a constant weight
at 105 °C. The difference in weight before
and after ignition provides an indirect measure
of biomass.
Quantitative Calculations
When needed, the relationship between the analyte and the analytical signal is given
by the stoichiometry of any relevant reactions. Calcula- tions are simplified, however, by applying
the principle of conservation of mass.
The most frequently encountered example
of a direct volatilization gravimetric analysis is the determination of a compound’s elemental composition.
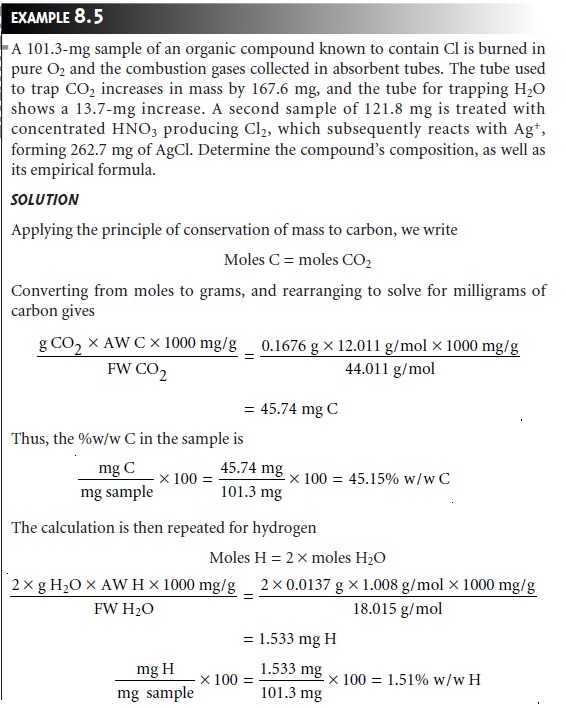
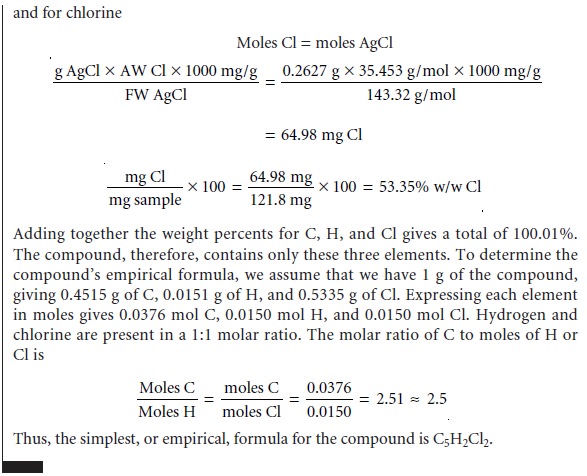
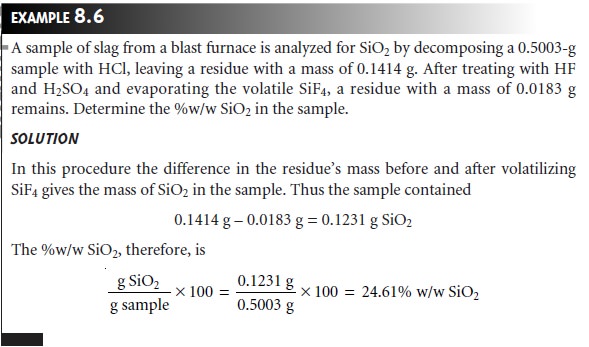
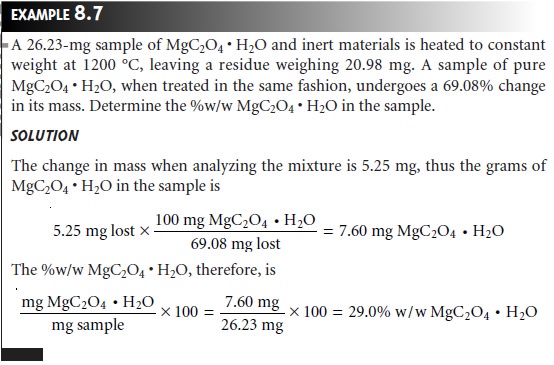
In an indirect volatilization gravimetric analysis, the change in the sample’s weight is proportional to the amount
of analyte. Note that in the following example it is not
necessary to apply
the conservation of mass to relate the
analytical signal to the
analyte.
Related Topics