Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Evaluating Volatilization Gravimetry
Evaluating Volatilization Gravimetry
The
scale
of
operation, accuracy, and precision of gravimetric volatilization
methods are similar
to that described for precipitation gravime- try. The sensitivity for a direct
analysis is fixed
by the analyte’s chemical form following combustion or volatilization. For an indirect analysis, however, sensi-
tivity can be improved by carefully choosing
the conditions for combustion or volatilization so that the
change in mass
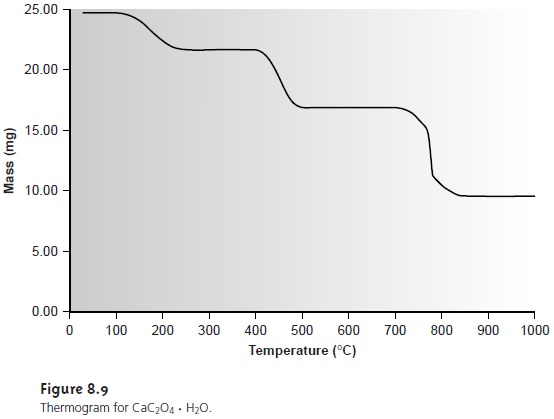
is as large as possible. For example, the thermogram
in Figure 8.9 shows that an indirect analysis for CaC2O4 H2O
based on the weight of the residue
following ignition at 1000 °C will be more sensitive than if the ignition was done 300 °C. Selectivity does not present a problem for direct volatilization gravimetric methods in which the analyte is a
gaseous product retained in an absorbent trap. A direct
analysis based on the residue’s weight following combustion or volatilization is possible when the residue only contains the analyte of interest. As noted earlier,
indirect analyses are only feasible when the residue’s change in mass results from the loss of a sin-
gle volatile product containing the analyte.
Volatilization gravimetric methods are time- and
labor-intensive. Equipment needs are few except when combustion gases
must be trapped
or for a thermogravi-
metric analysis, which
requires specialized equipment.
Related Topics