Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Overview of Gravimetry
Overview of Gravimetry
Before we look more closely
at specific gravimetric methods and their applications,
let’s take a moment to develop a broad survey
of gravimetry. Later, as you read through the different gravimetric methods, this survey will help you focus on their similarities. It is usually
easier to understand a new method of analysis when
you can see
its relationship to other similar
methods.
Using Mass as a Signal
We already indicated that in gravimetry we measure mass or
a change in mass. This suggests that there are at least
two ways to use mass as an analytical signal. We can,
of course, measure
an analyte’s mass
directly by placing
it on a balance
and recording its
mass. For example, suppose you are
to determine the total
suspended solids in water released
from a sewage-treatment facility. Sus- pended
solids are just that; solid
matter that has yet to settle out of its solution ma- trix. The analysis is easy. You collect a sample and pass it through a preweighed fil- ter
that retains the suspended solids.
After drying to remove any residual moisture, you weigh the filter.
The difference between
the filter’s original
mass and final
mass gives the mass of suspended solids. We call this a direct analysis
because the analyte itself is the object being weighed.
What if the analyte
is an aqueous ion, such as Pb2+? In this case we cannot iso- late
the analyte by filtration because
the Pb2+
is dissolved in the solution’s matrix. We can still measure the analyte’s mass, however, by chemically converting it to a solid
form. If we suspend a pair of Pt electrodes in our solution and apply a suffi-
ciently positive potential between them for
a long enough
time, we can
force the reaction
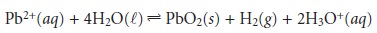
to go to completion. The
Pb2+ ion in solution oxidizes to PbO2 and deposits on the
Pt electrode serving as the anode. If we weigh
the Pt anode before and after applying the potential, the difference in the two measurements gives
the mass of PbO2 and, from the reaction’s stoichiometry, the mass of Pb2+. This also is a direct
analysis be- cause the
material being weighed
contains the analyte.
Sometimes it is easier to remove the
analyte and use
a change in mass as the
analytical signal. Imagine
how you would
determine a food’s
moisture content by a
direct analysis. One
possibility is to heat a sample of the food
to a temperature at which the water in the sample vaporizes. If we capture the vapor in a preweighed absorbent trap, then the change in the absorbent’s mass provides a di-
rect determination of the amount of water in the sample. An easier approach, however, is to weigh
the sample of food before
and after heating,
using the change in its mass as an indication of the amount
of water originally present. We call
this an indirect analysis since we determine the analyte by a signal
representing its disappearance.
The indirect determination of moisture content
in foods is done by difference.
The sample’s initial mass includes
the water, whereas
the final mass is measured after removing the water.
We can also determine an analyte indirectly without its ever being weighed. Again, as with the determination of Pb2+ as PbO2(s), we take
advantage of the analyte’s chemistry. For example, phosphite, PO33–, reduces Hg2+ to
Hg22+. In the presence of Cl– a solid precipitate of Hg2Cl2 forms.
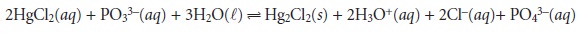
If HgCl2 is added in excess, each mole of PO33– produces one mole of Hg2Cl2. The precipitate’s mass, therefore, provides an indirect
measurement of the mass of PO33– present in the original
sample.
Summarizing, we can determine an analyte gravimetrically by
directly deter- mining its mass,
or the mass of a compound containing the analyte. Alternatively, we can determine an analyte indirectly by measuring a change in mass due
to its loss, or the mass
of a compound formed as the result
of a reaction involving the analyte.
Types of Gravimetric Methods
In the previous
section we used four examples
to illustrate the different ways that
mass can serve as an analytical signal.
These examples also
illustrate the four
gravi- metric methods of analysis. When the signal is the mass of a precipitate, we call the method precipitation gravimetry. The indirect determination of PO 3– by precipi- tating Hg2Cl2
is a representative example, as is the direct determination of Cl– by precipitating AgCl.
In electrogravimetry the analyte is deposited as a solid
film on one
electrode in an electrochemical cell. The oxidation of Pb2+, and
its deposition as PbO2 on a Pt anode is one example
of electrogravimetry. Reduction also may be used in electro-
gravimetry. The electrodeposition of Cu on a Pt cathode, for example, provides
a direct analysis for Cu2+.
When thermal or chemical energy
is used to remove a volatile species, we call the method
volatilization gravimetry. In determining the moisture content
of food, thermal energy
vaporizes the H2O. The amount of carbon in an organic
com- pound may be determined by using the chemical energy
of combustion to convert
C to CO2.
Finally, in particulate gravimetry the analyte is determined following its re- moval
from the sample
matrix by filtration or extraction. The determination of sus-
pended solids is one example
of particulate gravimetry.
Conservation of Mass
An accurate gravimetric analysis requires that
the mass of analyte present
in a sam- ple be proportional to the mass or change in mass serving as the analytical signal. For all gravimetric methods this proportionality
involves a conservation of mass. For gravimetric methods
involving a chemical
reaction, the analyte
should partici- pate in only one
set of reactions, the stoichiometry of which indicates how the pre- cipitate’s mass relates to the analyte’s mass. Thus, for the analysis
of Pb2+
and PO 3– described earlier, we can write the following conservation equations
Moles Pb2+ = moles PbO2
Moles PO33– = moles Hg2Cl2
Removing the analyte
from its matrix
by filtration or extraction must be complete. When true, the analyte’s mass can always
be found from
the analytical signal;
thus, for the determination of suspended solids
we know that
Filter’s final mass – filter’s initial
mass = g suspended solid
whereas for the
determination of the
moisture content we have
Sample’s initial mass –
sample’s final mass = g H2O
Specific details, including worked examples.
Why Gravimetry Is Important
Except for particulate gravimetry, which is the most trivial
form of gravimetry, it is entirely possible
that you will never use gravimetry after you are finished with this
course. Why, then, is familiarity with gravimetry still
important? The answer
is that gravimetry is one of only a small number
of techniques whose
measurements re- quire only base SI units, such as mass and moles,
and defined constants, such as Avogadro’s number and the mass of 12C.* The result of an analysis
must ultimately be traceable to methods, such
as gravimetry, that
can be related
to fundamental physical properties.1 Most analysts
never use gravimetry to validate their
methods. Verifying a method
by analyzing a standard reference material, however, is com-
mon. Estimating the composition of these materials often involves a gravimetric
analysis.
Related Topics