Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Precipitation Gravimetry: Evaluating Precipitation Gravimetry
Evaluating Precipitation Gravimetry
Scale of Operation
The scale
of operation for precipitation gravimetry is governed by the sensitivity of the balance
and the availability of sample.
To achieve an accu-
racy of ±0.1% using an analytical balance
with a sensitivity of ±0.1 mg, the precipi-
tate must weigh at least
100 mg. As a consequence, precipitation gravimetry is usu-
ally limited to major or minor analytes, and macro or meso samples.
The analysis of trace level
analytes or micro
samples usually requires a microanalytical balance.
Accuracy
For
macro–major samples, relative
errors of 0.1–0.2%
are routinely achieved. The
principal limitations are
solubility losses, impurities in the precipitate, and the loss of precipitate during
handling. When it is difficult to obtain a precipitate
free from impurities, an empirical
relationship between the precipitate’s mass and
the mass of the analyte
can be determined by an appropriate standardization.
Precision
The
relative
precision of precipitation gravimetry depends on the
amount of sample and precipitate involved. For smaller
amounts of sample or
precipitate, relative precisions of 1–2 ppt are routinely obtained. When working with larger amounts of sample or precipitate, the relative precision
can be ex- tended to several parts
per million. Few
quantitative techniques can
achieve this level of precision.
Sensitivity
For any precipitation gravimetric method, we can write
the following general equation
relating the signal
(grams of precipitate) to the absolute
amount of analyte in the sample
Grams precipitate = k x grams of
analyte 8.13
where k, the method’s sensitivity, is determined by the stoichiometry between the
precipitate and the analyte. Note that equation
8.13 assumes that a blank
has been used to correct the signal for the reagent’s contribution to the precipitate’s mass.
Consider, for example,
the determination of Fe as Fe2O3. Using
a conservation of mass for Fe we write
2 x moles Fe2O3 = moles Fe
Converting moles to grams and
rearranging yields an equation in the form of 8.13
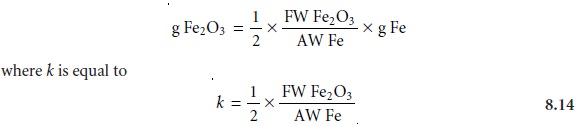
As can be seen from
equation 8.14, we may improve
a method’s sensitivity in two ways. The most obvious
way is to increase the ratio of the precipitate’s molar mass to that of the analyte. In other words,
it is desirable to form a precipitate with as large a formula weight
as possible. A less obvious
way to improve the calibration sensitivity is indicated by the term of 1/2 in equation
8.14, which accounts
for the stoichiometry between
the analyte and precipitate. Sensitivity also may be improved
by forming precipitates containing fewer
units of the
analyte.
Selectivity
Due to the chemical nature of the
precipitation process, precipitants are usually not selective for a single
analyte. For example, silver is not
a selective precipitant for chloride because
it also forms
precipitates with bromide
and iodide. Consequently, interferents are often a serious problem
that must be considered if accurate results are to be obtained.
Time, Cost, and Equipment
Precipitation gravimetric procedures are time-intensive and rarely practical when
analyzing a large
number of samples. However, since much of
the time invested in precipitation gravimetry does not require
an analyst’s immedi- ate supervision, it may be a practical alternative when working with only a few sam- ples. Equipment needs are few (beakers, filtering devices, ovens
or burners, and bal-
ances), inexpensive, routinely available in most
laboratories, and easy
to maintain.
Related Topics