Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Theory and Practice of Precipitation Gravimetry: Solubility Considerations
Theory and Practice
A precipitation gravimetric analysis must have several important
attributes. First, the precipitate must be of low solubility, high purity, and of known
composition if its mass
is to accurately reflect the
analyte’s mass. Second,
the precipitate must
be in a form
that is easy
to separate from
the reaction mixture. The theoretical and
exper- imental details of precipitation gravimetry are reviewed in this section.
Solubility Considerations
An accurate precipitation gravimetric method requires that the precipitate’s solubility be minimal. Many total analysis
techniques can rou- tinely be performed with
an accuracy of better than
±0.1%. To obtain
this level of accuracy, the isolated precipitate must account for at least
99.9% of the analyte. By extending this requirement to 99.99% we ensure that accuracy is not limited
by the precipitate’s solubility.
Solubility losses are minimized
by carefully controlling the composition of the
solution in which the precipitate forms. This, in turn, requires
an understanding of the
relevant equilibrium reactions affecting the precipitate’s solubility. For example, Ag+ can be determined gravimetrically by adding Cl– as a precipitant, forming
a precipitate of AgCl.
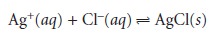 8.1
8.1
If
this is the
only reaction considered, we would falsely
conclude that the
precipi- tate’s solubility, SAgCl, is given by
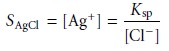 8.2
8.2
and that solubility losses may be minimized by adding a large excess
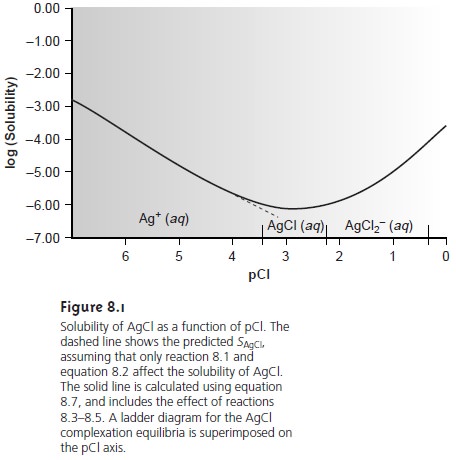
of Cl–. In fact, as shown
in Figure 8.1, adding a large excess
of Cl– eventually increases the precipi- tate’s solubility.
To
understand why AgCl shows a more complex
solubility relationship than that suggested by equation
8.2, we must recognize that Ag+ also forms a series
of soluble chloro-complexes
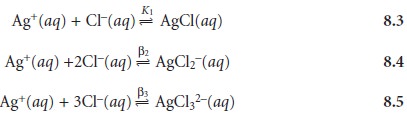
The
solubility of AgCl,
therefore, is the sum of the equilibrium concentrations for
all soluble forms of Ag+.
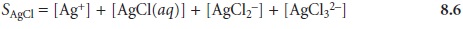
Substituting the equilibrium constant expressions for reactions
8.3–8.5 into equation 8.6 defines the solubility of AgCl in terms of the
equilibrium concentration of Cl–
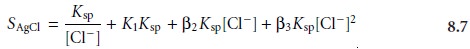
Equation 8.7 explains the solubility curve for AgCl shown in Figure 8.1. As Cl– is added to a solution of Ag+, the solubility of AgCl initially decreases because of re- action 8.1. Note that under these conditions, the final three terms in equation 8.7 are small, and that equation 8.1 is sufficient to describe the solubility of AgCl. In- creasing the concentration of chloride, however, leads to an increase in the solubil- ity of AgCl due to the soluble chloro-complexes formed in reactions 8.3–8.5.*
Clearly the equilibrium concentration of chloride
is an important parameter if the
concentration of silver is to be determined gravimetrically by precipitating AgCl. In particular, a large excess
of chloride must be avoided.
Another important parameter that may affect
a precipitate’s solubility is the pH of
the solution in which the precipitate forms.
For example, hydroxide precipitates, such as Fe(OH)3, are more soluble
at lower pH levels at which the concentration of OH– is small.
The effect of pH on solubility is not limited
to hydroxide precipitates, but also affects
precipitates containing basic or acidic ions. The solubility of Ca3(PO4)2 is pH-dependent because
phosphate is a weak base.
The following four reactions, therefore, govern the
solubility of Ca3(PO4)2.
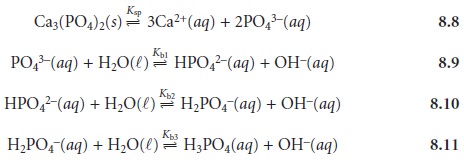
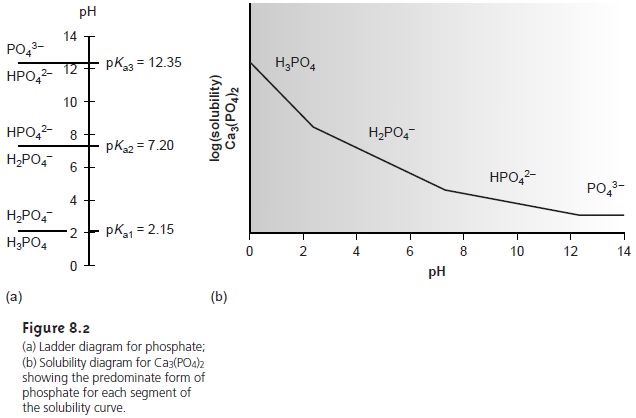
Depending on the solution’s pH, the predominate phosphate species is either PO43–, HPO42–, H2PO4–, or H3PO4. The ladder diagram
for phosphate, shown
in Figure 8.2a, provides a
convenient way to evaluate the pH-dependent solubility of phos- phate precipitates. When the pH is greater
than 12.4, the predominate phosphate species is PO43–, and the solubility of Ca3(PO4)2 will
be at its minimum because only reaction 8.8 occurs
to an appreciable extent (see Figure 8.2b).
As the solution becomes more acidic,
the solubility of Ca3(PO4)2 increases due to the contributions
of reactions 8.9–8.11.
Solubility can often be decreased by using a nonaqueous solvent. A precip- itate’s solubility is generally greater in aqueous solutions because of the ability of water molecules to stabilize ions through solvation. The poorer solvating ability of nonaqueous solvents, even those that are polar, leads to a smaller solu- bility product. For example, PbSO4 has a Ksp of 1.6 x 10–8 in H2O, whereas in a 50:50 mixture of H2O/ethanol the Ksp at 2.6 x 10–12 is four orders of magnitude smaller.
Related Topics