Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Variation of Ionization Energy in the periodic Table
Ionization Energy: (Ionization Potential)
In modern terminology, ionization energy is known as ionization enthalpy. The energy required to remove an electron from an atom is known as ionization enthalpy (IE). The
first ionization enthalpy may be defined as the amount of energy required to
remove the most loosely bound electron from the isolated gaseous atom.
Atom (g)+Energy->Positive
ion (g) + Electron
For
example,
Li (g) + 520 kJ mol-1 -> Li+ (g) + e-
Ionization enthalpy is also called ionization potential because it is
measured as the amount of potential required to remove the most loosely held
electron from the gaseous atom. It is expressed in terms of either kJ/mol or
electron Volts/atom.
If a second electron is to be removed from the
same element the energy required will be higher than that required for removal
of the first electron because it is more difficult to remove an electron from a
positively charged species than from a neutral atom.
Li+ (g) + 7297 kJ mol-1 -> Li2+ + e-
Similarly the third ionization enthalpy will be
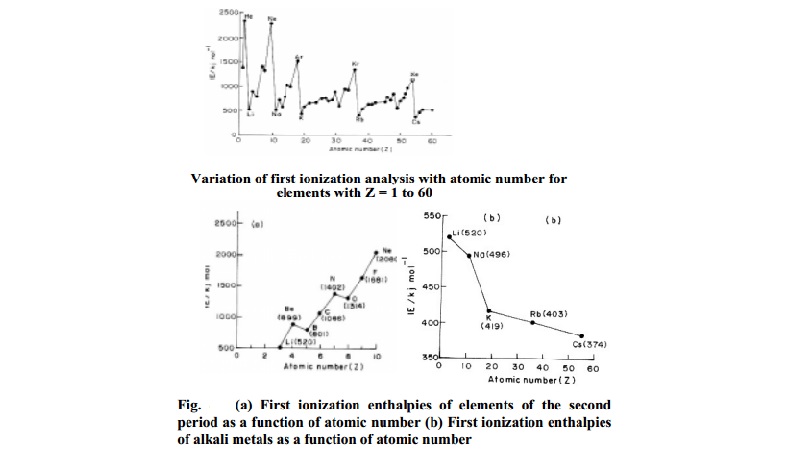
higher than the second and so on. Fig. shows a plot of first ionization
enthalpy of some elements.
Variation of Ionization Energy in the periodic
Table
It is seen from the Fig. that the ionization enthalpy of an element
depends on its electronic configuration. Ionization potentials of noble gases
are found to be maximum and those of alkali metals are found to minimum. The
high values of noble gases are due to completely filled electronic
configurations in their outermost shells and the low values of alkali metals
are due to their large size and a single electron in the outermost shell.
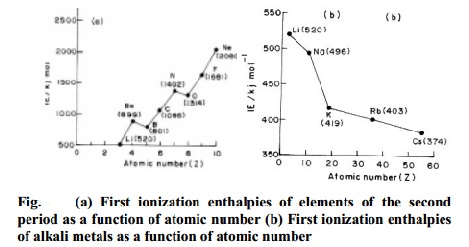
The ionization potentials increases from left to
right in a period. This trend can be explained in terms of increase in nuclear
charge and decrease in size from left to right in a period. Generally the first
ionization enthalpy decreases down a group in the periodic table. As we move
down the group, the outer electrons, which are to be removed, are farther from
the nucleus and there is an increasing screening of the nuclear charge by the
electrons in the inner shells. Consequently the removal of electrons becomes
easier down the group.
Factors Influencing Ionization Enthalpy
The ionization enthalpy of an atom depends on the following factors.
(i) Size
of the atom
As the distance between the electron and the nucleus increases, i.e., as
the size of the atom increases, the outermost electrons are less tightly held
by the nucleus. Thus, it becomes easier to remove an outermost electron. Thus
ionization enthalpy decreases with increases in atomic size.
(ii) Charge on the nucleus
Ionization enthalpy increases with increase in
nuclear charge because of the increase in the attractive force between the
nucleus and the electron.
(iii) Screening effect of inner electrons
Ionization enthalpy decreases when the shielding
effect of inner electrons increases. This is because when the inner electron
shells increases, the attraction between the nucleus and the outermost electron
decreases.
(iv) Penetration effect of electrons
The penetration power of the electrons in various orbitals decreases in
a given shell (same value of n) in the order: s>p>d>f. Since the
penetration power of s-electron towards the nucleus is more, it will be closer
to the nucleus and will be held firmly. Thus, for the same shell, the
ionization enthalpy would be more to remove the s-electrons in comparison with
the p-electron which in turn would be more than that for d-electron and so on.
(v) Effect of half-filled and completely filled
sub-levels
If an atom has half-filled or completely filled sub-levels, its
ionization enthalpy is higher than that expected normally from its position in
the periodic table. This is because such atom, have extra stability and hence
it is difficult to remove electrons from these stable configurations.
Related Topics