Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Atomic and ionic radii
Atomic and ionic radii
The size of an atom can be visualized from its atomic radius. The term
atomic or ionic radius is generally defined as the distance between the centers
of the nucleus and the outermost shell of electrons in an atom or ion. For
example, the atomic radius of hydrogen atom is equal to 74/2 pm = 37 (bond
distance in hydrogen molecule (H2) is 74pm).
Atomic and
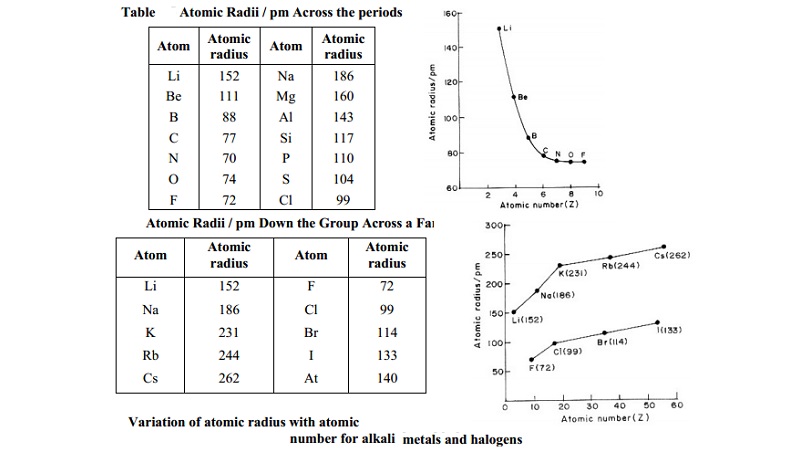
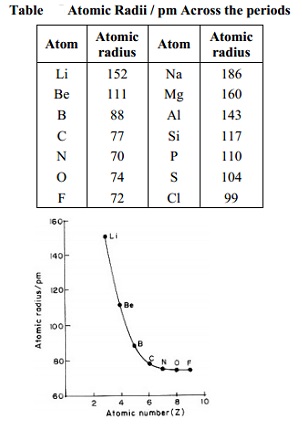
ionic radii both decrease from left to right across a period in the periodic
table when we consider only normal elements, e.g. in the elements of 2nd
period the covalent radii decrease as we move from Li to F as shown below:
Elements
of 2nd period :
Li
Be B C N
O F
Covalent radii Values decreasing from Li to F
Thus in any period the alkali metals (that are present at the extreme left
of the periodic table) have the largest size while the halogens (that are
present at the extreme right, excluding the zero group elements) have the
smallest size.
Explanations
We know
that as we proceed from left to right in a period, the electrons are added to
the orbitals of the same main energy level. Addition of different electrons to
the same main energy level puts the electrons, on the average, no farther from
the nucleus and hence the size can not be increased. But with the addition of
each electron, the nuclear charge (i.e. atomic number) increases by one. The
increased nuclear charge attracts the electrons more strongly close to the
nucleus and thus decreases the size of the atoms.
Atomic Radii / pm Across the periods
Atom Atomic Atom Atomic
radius radius
Li 152 Na 186
Be 111 Mg 160
B 88 Al 143
C 77 Si 117
N 70 P 110
O 74 S 104
F 72 Cl 99
(b) In a group
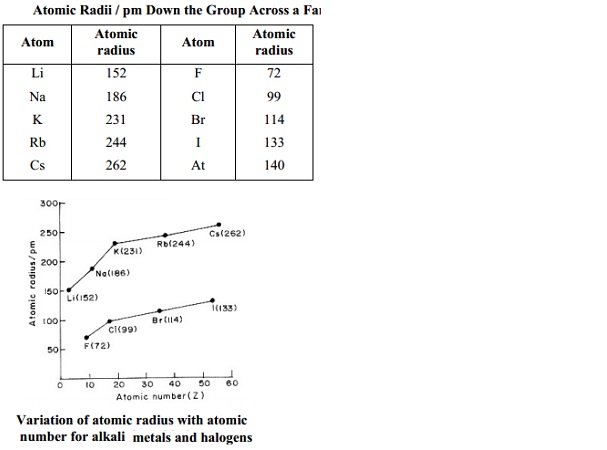
On moving
down a group of regular elements both atomic and ionic radii increase with
increasing atomic number, e.g. in the elements of IA Group both covalent and
ionic radii of M+ ions increase when we pass from Li to Cs
Elements of IA Group : Li
Na K Rb Cs
Covalent radii/Ionic radii Values increasing from Li to Cs
Explanation
On proceeding downwards in a group the electrons are added to higher
main energy levels, which are, on the average, farther from the nucleus. This
effect decreases the electrostatic attraction between the nucleus and the
valence-shell electrons and this decreased electrostatic attraction increases the
atomic and ionic radii.
Table : Atomic Radii /
pm Down the Group Across a Family
Atom Atomic Atom Atomic
radius radius
Li 152 F 72
Na 186 Cl 99
K 231 Br 114
Rb 244 I 133
Cs 262 At 140
When we find some atoms and ions, which contain the same number of
electrons, we call them isoelectronic.
For example, O2-, F-, Na+ and Mg2+
have the same number of electrons (10). Their radii would be different because
of their different nuclear charges. The cation with the greater positive charge
will have a smaller radius because of the greater attraction of the electrons
to the nucleus. Anions with the greater negative charge will have the larger
radius. In this case, the net repulsion of the electrons will outweigh the
nuclear charge and the ion will expand in size.
Example
Which of
the following species will have the largest and the smallest size Mg, Mg2+,
Al, Al3+?.
Solution
Atomic radii decrease across a period. Cations are smaller than their
parent atoms. Among isoelectronic ions, the one with the large positive nuclear
charge will have a smaller radius.
Hence the largest species is Mg; the smallest one is Al3+
The size of an anion greater while that of the
cation is smaller than that of its parent atom, e.g. F- (=1.36
Å)>F(=0.72 Å); Cl -(=1.81 Å)>Cl(=0.99Å); Na +(=0.95Å)<Na(=1.90Å);
Ca 2+(=0.99 Å)<Ca(=1.97Å).
Explanation
Let us consider the radii of Na, Na+,
Cl and Cl-. The reason of the fact that Na+ ion is
smaller than Na atom is that Na+ ion has 10 electrons (Na+->1s2,2s2p6)
while Na atom has 11electrons (Na ->1s2,2s2p6,3s1). The nuclear
charge (charge on the nucleus) in each case is the same, i.e. equal to +11
(atomic number of Na). This nuclear charge of +11 can pull 10 electrons of Na+
ion inward more effectively than it can pull a greater number of 11 electrons
of Na atom. Thus Na+ ion is smaller than Na atom.
The reason
why Cl- ion is bigger than Cl atom can also be explained on a
similar basis. The Cl- ion has 18 electrons (Cl-->1s2,2s2p6,3s2p6)
while Cl atom has only 17 electrons (Cl->1s2,2s2p6,3s2p5).
The nuclear charge in each case is +17, which cannot pull 18 electrons of Cl-
ion as effectively as it can pull 17 electrons of Cl atom inward. Thus Cl-
ion is bigger than Cl atom.
Related Topics