Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Valence Shell Electron Pair Repulsion Theory (VSEPR) Theory
Valence Shell Electron Pair Repulsion Theory
(VSEPR) Theory
Molecules exist in different shapes. Many of the physical and chemical
properties of molecules arise due to different shapes of the molecules.
Some of the common geometrical shapes found among the molecules are:
linear, trigonal, planar, tetrahedral, square planar, trigonal bipyramidal,
square pyramidal, octahedral, pentagonal-bipyramidal etc. The VSEPR theory
provides a simple treatment for predicting the shapes of polyatomic molecules.
The theory was originally proposed by Sigdwick and Powell in 1940. It was
further developed and modified by Nyholm and Gillespie (1957).
The basic
assumptions of the VSEPR theory are that:
i.
Pairs of electrons in the valence shell of a
central atom repel each other.
ii.
These pairs of electrons tend to occupy
positions in space that minimize repulsions and maximise the distance of
separation between them.
iii.
The valence shell is taken as a sphere with
electron pairs localising on the spherical surface at maximum distance from one
another.
iv.
A multiple bond is treated as if it is a single
electron pair and the two or three electron pairs of a multiple bond are
treated as a single super pair.
v.
Where two or more resonance structures can
depict a molecule the VSEPR model is applicable to any such structure.
It is convenient to divide molecule into two
categories (i) molecules in which the central atom has no lone pairs of
electrons and (i) molecules in which the central atom has one or more lone
pairs.
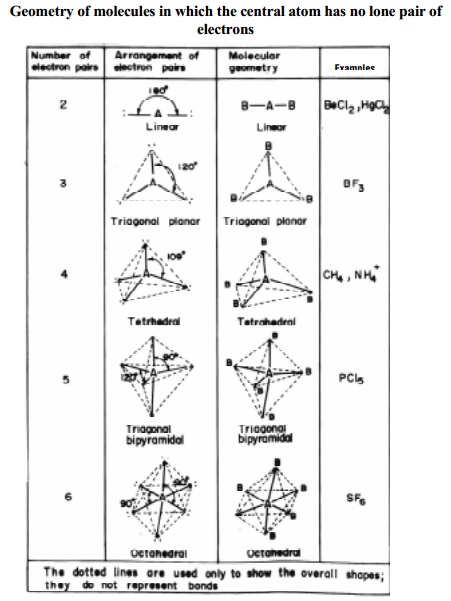
Table
shows the different geometries of molecules or ions with central atom having no
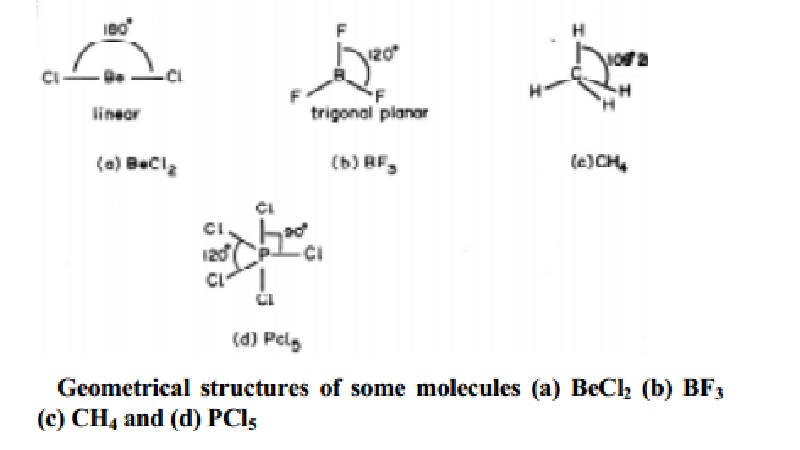
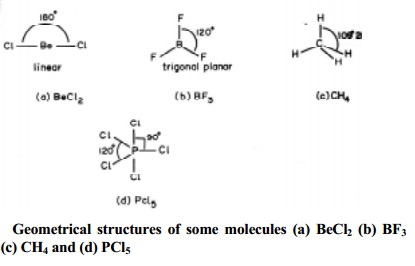
lone pair of electrons and represented by general type ABx. In compounds of AB2,
AB3, AB4, AB5, AB6, types the arrangement of electron pairs (bonded pairs) as
well as the B atoms around the central atom A are, linear, trigonal planar,
tetrahedral, trigonal-bipyramidal and octahedral respectively. Such
arrangements are present in BeCl2 (AB2); BF3
(AB3); CH4 (AB4) and PCl5 (AB5)
molecules with geometries as shown below in Fig..
In case of molecules with the central atom having one or more lone pairs
VSEPR treatment is as follows: In these type of molecules, both lone pairs and
bond pairs of electrons are present. The lone pairs are localised on the
central atom, and bonded pairs are shared between two atoms. Consequently, the
lone pair electrons in a molecule occupy more space as compared to the bonding
pair electrons. This causes greater repulsions between lone pairs of electrons
as compared to the lone pairs of electrons to the lone pair (lp) - bonding pair
and bonding pair - bonding pair repulsions (bp).
The descending order of repulsion interaction is
lp - lp
> lp - bp > bp - bp
These
repulsion effects cause deviations from idealised shapes and alterations in the
predicted bond angles in molecules.
Examples :
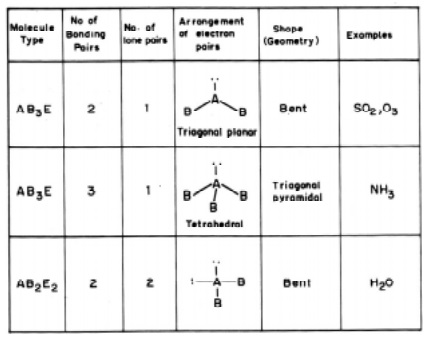
In sulphur dioxide molecule there are three electron pairs on the S atom. The
overall arrangement is trigonal planar. However, because one of the three
electron pairs is a lone pair, the SO2 molecule has a `bent' shape and due to
the lp - lp repulsive interactions the bond angle is reduced to 119.5 from the value of 120 deg.
In the
ammonia (NH3) molecule, there are three bonding pairs and one lone pair of
electrons. The overall arrangement of four electron pairs is tetrahedral. In NH3,
one of the electron pairs, on nitrogen atom is a lone pair, so the geometry of
NH3 is pyramidal (with the N atom at the apex of the pyramid). The
three N-H bonding pairs are pushed closer because of the lp-bp repulsion and
the HNH angle gets reduced from 109O28' (which is the tetrahedral angle) to 107 O.
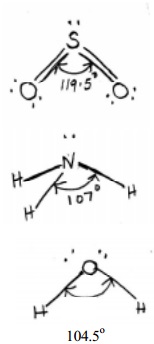
The water
H2O molecule, oxygen atom contains two bonding pairs and two lone pairs of
electrons. The overall arrangement for four electron pairs is tetrahedral, but
the lp - lp repulsions being greater than lp-bp repulsions in H2O.
The HOH angle is reduced to 104.5 O than 109 O 28'. The
molecule has a bent shape.
The
molecule SF6 belongs to AB6 type consisting of 6 bp of electrons around the
central sulphur atom. The geometrical arrangement will be a regular octahedral.
Related Topics