Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Types of Isomerism: Structural and Stereo isomerism
ISOMERISM
Compounds having the same chemical formula but different physical and chemical properties due to the different structural arrangements are called isomers. This phenomenon is known as isomerism.
Coordination compounds exhibit two major types of
isomerism, namely
(A) structural isomerism and (B) stereoisomerism (space
isomerism). Each of these is further
classified as shown below.
A) Structural isomerism
a) Coordination isomerism
c) Hydrate or Solvate isomerism
e) Ligand Isomerism
b) Ionisation isomerism
d) Linkage isomerism
B)
Stereoisomerism
a) Geometrical isomerism
b) Optical isomerism
1
A) Structural isomerism
a) Coordination isomerism
In a bimetallic complex, both complex cation and complex
anion may be present. In such a case the distribution of ligands between the
two coordination spheres can vary,
giving rise to isomers called the coordination isomers. This phenomenon is called coordination isomerism. This
isomerism is illustrated by the following
pairs of complexes where the complex cation and anion contain different metal centres.
1.
[CoIII(NH3)6] [Cr(CN)6] and [CrIII(NH3)6] [CoIII(CN)6]
Hexammine hexacyano Hexamine
hexacyano
cobalt(III)
chromate(III) chromium (III) cobaltate (III)
2. [PtII(NH3)4] [CuCl4] and [Cu(NH3)4] [PtCl4]
Tetraammine Tetrachloro Tetraammine Tetrachloro
platinum (II) cuparate (II) copper (II) platinate (II)
b) Ionisation isomerism
Coordination compounds having the same molecular formula
but forming different ions in solution are called ionisation isomers. This
property is known as ionisation isomerism.
An example of this type of isomerism is furnished by the
red-violet,
[Co(NH3)5Br]SO4 [Co(NH3)5 SO4]Br
pentaamminebromocobalt(III) sulphate pentaamminesulphatocobalt
(III) bromide
The red-violet isomer yields
sulphate ion and the red isomer furnishes bromide
ion in solution.
[Co(NH3)4Cl2]NO2 and [Co(NH3)4 NO2Cl]Cl
Tetraamminedichlorocobalt(III) nitrite Tetraamminechloronitrocobalt(III)
chloride
[Co(NH3)5NO3]SO4 and [Co(NH3)5 SO4]NO3
pentaamminenitratocobalt(III) sulphate pentaamminesulphatocobalt(III)
nitrate
c) Hydrate isomerism or Solvate isomerism
The best known examples of this type of isomerism occurs
for chromium chloride "CrCl3.6H2O" which
may contain 4, 5, (or) 6 coordinated water
molecules.
1. [Cr(H2O)4Cl2]Cl.2H2O - Bright green
Tetraaquadichlorochromium(III) chloride dihydrate
2. [Cr(H2O)5Cl]Cl2.H2O - grey-green
Pentaaquachlorochromium(III) chloride monohydrate
3. [Cr(H2O)6]Cl3 - Violet
Hexaaquachromium(III) chloride
These isomers have very different chemical properties
and on reaction with AgNO3 to test for Cl- ions, would find 1,2, and 3 Cl- ions in solution respectively.
d. Linkage isomerism
Linkage isomerism occurs with ambidentate ligands. These
ligands are capable of coordinating in
more than one way. The best known cases involve the monodentate ligands SCN-/NCS- and NO2-/ONO-
For example
[Co(NH3)5ONO]Cl2 the
nitrito isomer - red colour
pentaamminenitritocobalt(III) chloride - O attached
[Co(NH3)5 NO2]Cl2 the nitro isomer - yellow colour
pentaamminenitrocobalt(III) chloride - N attached
e) Ligand isomerism
Ligand isomerism arises from the presence of ligands
which can adopt different isomeric
forms. An example is provided by diaminopropane, which may have the amine groups in the terminal (1,3-)
positions or in the 1,2-positions.
H2N - CH2 - CH2 - CH2 - NH2
2 Stereoisomerism (space isomerism)
Consider two compounds containing the same ligands
attached to the same central metal ion, but the arrangement of ligands in space
about the central metal ion are different,
then these two compounds are said to be stereoisomers and this phenomenon is known as stereoisomerism. There are
two different types of stereoisomerism.
a) Geometrical isomerism or b) Optical isomerism.
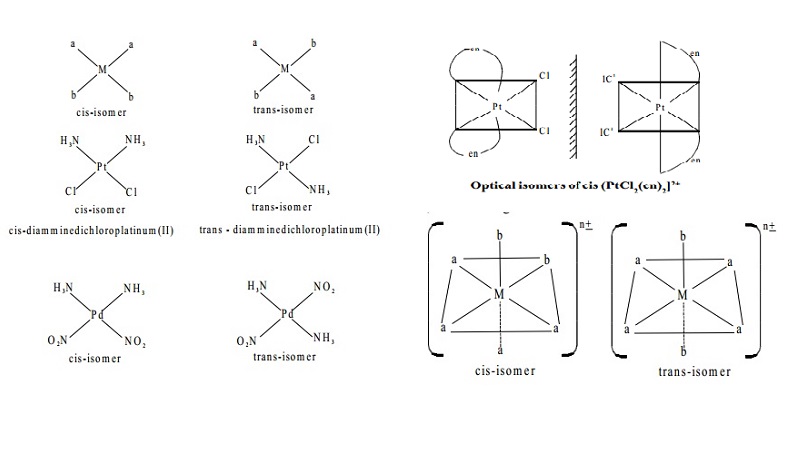
a) Geometrical (or) cis-trans isomerism
Geometric isomers are possible for both square planar
and octahedral
complexes, but not tetrahedral. In a cis-isomer two
identical (or) similar groups are adjacent
to each other whereas in a trans-isomer they are diametrically opposite to each other.
Square
planar complexes of the type [Ma2b2]n+ where
a and b are monodentate ligands, exist as
cis and trans-isomers as shown below. Example of this type of complexes are [Pt (NH3)2 Cl2] and [Pd(NH3)2 (NO2)2]. The cis- trans isomers of these compounds are represented as
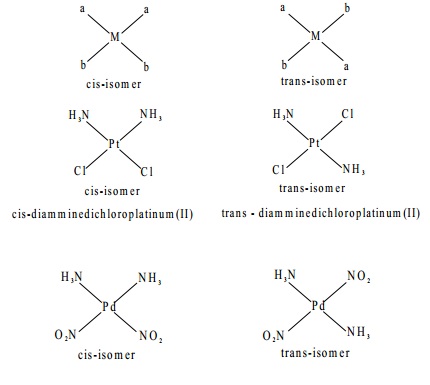
In the octahedral complex, the different coordination positions are numbered as shown below
Along the twelve edges of the octahedron, there are
twelve cis positions.
They are (1,2) (1,3) (1,4) (1,5) (2,6) (3,6) (4,6) (5,6)
(3,4) (4,5) (2,5) and (2,3). In order to
avoid confusion, generally it is assumed that the 1,2 positions are cis-positions. There are three trans positions; they
are (1,6) (2,4) and (3,5). Normally it is
taken that 1,6 positions are trans-positions in order to avoid confusion.
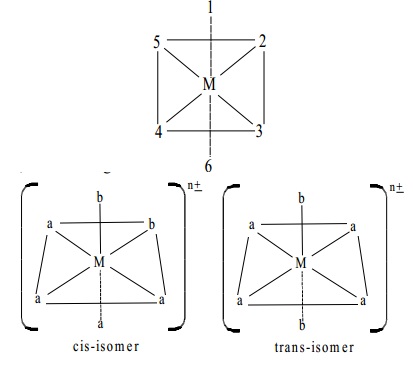
An octahedral complex of the type [Ma4b2] where a and
b are monodentate
ligands, exists as two geometrical Isomers:
A specific example for such Isomerism is [Co(NH3)4 Cl2]+ which
exists as
two geometrical isomers.
The octahedral complex are of the type [M(AA)2a2]n± where (AA) is a
symmetrical bidentate ligand such as ethylenediamine H2N-CH2-CH2-NH2and 'a' is a
monodentate ligand. A specific example for this is [Co(H2N-CH2-CH2-
NH2)2 Cl2]+
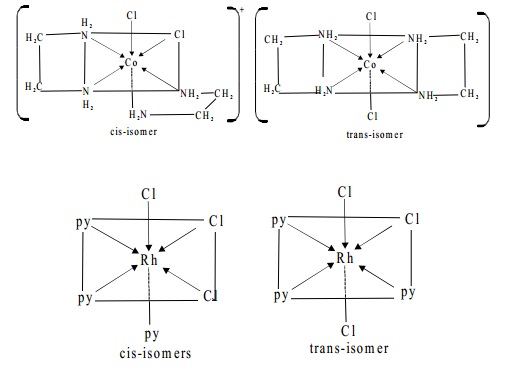
The
octahedral complex of the type, [Ma3b3]n±,
where a and b are monodentate ligands also
exist as geometrical isomers, For example, [Rh(py)3 Cl3]
exist as cis-(1,2,3 trichlorocomplex) and trans-(1,2,6-trichloro complex) isomers as represented below
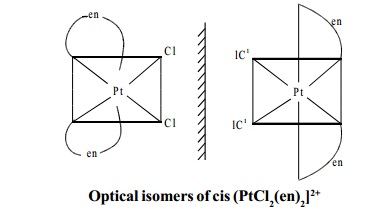
b) Optical Isomerism
This is a phenomenon in which certain organic or
inorganic compounds have the property
of rotating plane polarised light. The compounds which exhibit this property
are called optical isomers. The optical isomers of a compound have identical physical and chemical properties. The only
distinguishing property is that the isomers rotate the plane of polarised light
either to the left or right. In a coordination
compound of type [PtCl2(en)2]12+,
two geometrical isomers are
possible. They are cis and trans. Among these two
isomers, cis isomer shows optical
activity because the whole molecule is asymmetric.
Related Topics