Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Theory of Hybridisation
Theory of Hybridisation
The failures of VB theory based on pure orbital
overlaps are explained agreeably based on the concept of hybridisation of
orbitals or mixing up of orbitals. There are three major processes that are
considered to occur in hybridisation of orbitals. These are:
1. Promotion of electrons to higher or similar energy levels
2.
Mixing up of various s,p,d,f orbitals to form
the same number of new orbitals and
3.
Stabilisation of the molecule through bond
formations involving hybrid orbitals by release of certain amount of energy
which compensates the energy requirement in the electron promotion process.
Promotion (Excitation) of Electrons
Atoms of elements like Beryllium, Boron and Carbon have electronic
configuration as,
Be
(At. no : 4) : 1s22s2
(At. no :
5) : 1s22s22px1
(At. no : 6) : 1s22s22px12py1
According
to VB theory, Beryllium is expected to behave like a noble gas due to its
filled shells, which in practice forms a number of compounds like BeF2 and BeH2
proving its bivalency. In case of Boron VB theory predicts univalency due to
the presence of one unpaired electron but in practice Boron is trivalent since
compounds as BCl3, BH3 etc. are found.
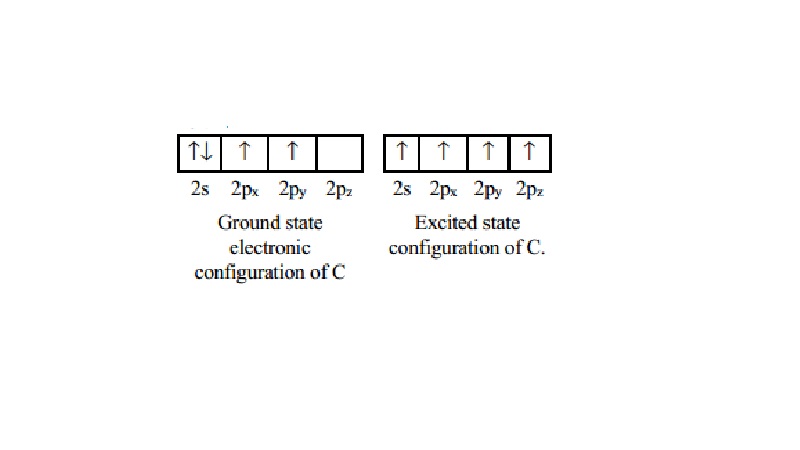
The stable
state (Ground State) electronic configuration of C is (2s22px12py1).
Electronic configuration of C suggests only bivalency. But carbon forms over a
million compounds in all of which carbon is tetravalent. This suggests only
tetravalency. This deficiency is overcome by allowing for promotion (or) the excitation
of an electron to an orbital of higher energy. Although for electron promotion
energy is needed, if that energy is recovered back during a covalent bond
formation, or by a bond with a greater strength or by many number of bonds
formation, then the electron promotion becomes energetically allowed and
assumed to take place initially. In carbon, promotion of an electron to an
orbital which is close to itself with an empty orbital of only slightly higher
energy which is the 2pz orbital can take place. Then the electron pair is
unpaired itself by absorbing the required energy available by the atom from its
surrounding and one of the electrons in the original orbital 2s or 2p shifts to
the empty higher energy orbital.
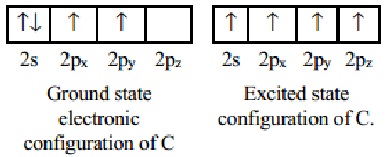
Thus promotion of an electron leads to four
unpaired electron in the excited state electronic configuration of carbon atom.
Each electron can now be utilised to form a covalent bond by sharing an
electron coming from the combining atom. The four 'Sig-ma' covalent bonds are
possible, each with
equivalent strength and overlapping tendency. Further, chemical and physical
evidences reveal the four bonds of carbon to be equivalent and that they are
tetrahedrally oriented. The promotion of an electron from 2s to 2p orbital
leads to four half filled orbitals which can form four bonds leading to greater
energy lowering. This energy is more than the initial energy required for the
promotion of 2s electron to 2p orbital.
Hybridisation (mixing of orbitals)
After an electron promotion the 4 electrons are
not equivalent, since one of them involves with an s orbital while the other
three involve with p orbitals. To explain the equivalence of the four bonds,
the concept of hybridisation is introduced.
Dissimilar orbitals like s,p,d with one or many
numbers, with nearly the same energy on the same atom may combine or mix
completely to form an equal number of equivalent energy new orbitals with
properties of their own. This is called as
hybridisation of orbitals. The new orbitals formed are known as hybrid
orbitals and these orbitals possess the properties of the pure orbitals that
are mixed to form them. The hybrid orbitals of an atom are symmetrically
distributed around it in space. Essentially, mixing up of orbitals to form new
orbitals explains the different geometries of many compounds like CH4, SF6 etc.
Related Topics