Accuracy and Precision, Errors in Measurement, Error Analysis, Propagation of errors - Theory of Errors | 11th Physics : UNIT 1 : Nature of Physical World and Measurement
Chapter: 11th Physics : UNIT 1 : Nature of Physical World and Measurement
Theory of Errors
THEORY OF ERRORS
The
foundation of all experimental science and technology is measurement. The
result obtained from any measurement will contain some uncertainty. Such an
uncertainty is termed error. Any calculation made using the measured values will also have an
error. It is not possible to make exact measurements in an experiment. In
measurements, two different terms accuracy and precision are used and need to
be distinguished at this stage. Accuracy refers to how far we are from the true
value, and precision refers to how well we measure.
Accuracy and Precision
Let
us say, you know your true height is exactly 5′9″. You first measure your height with
a yardstick and get the value 5′0″. Your measurement is hence not
accurate. Now you measure your height with a laser yardstick and get 5′9″ as the value. Now your measurement
is accurate. The true value is also called theoretical value. The level of
accuracy required for each application varies greatly. Highly accurate data can
be very difficult to produce and compile. For example, if you consistently
measure your height as 5′0″ with a yard stick, your
measurements are precise. The level of precision required for different
applications vary to a great extent. Engineering projects such as road and
utility construction require very precise information measured to the
millimeter or one-tenth of an inch.
If
a measurement is precise, that does not necessarily mean that it is accurate.
However, if the measurement is consistently accurate, it is also precise.
For
example, if the temperature outside a building is 40oC as measured
by a weather thermometer and if the real outside temperature is 40oC,
the thermometer is accurate. If the thermometer consistently registers this
exact temperature in a row, the thermometer is precise.
Consider
another example. Let the temperature of a refrigerator repeatedly measured by a
thermometer be given as 10.4oC, 10.2oC, 10.3oC,
10.1oC, 10.2oC, 10.1oC, 10.1oC,
10.1oC. However, if the real temperature inside the refrigerator is
9oC, we say that the thermometer is not accurate (it is almost one
degree off the true value), but since all the measured values are close to 10oC,
hence it is precise.
A visual example:
Target
shooting is an example which explains the difference between accuracy and
precision. In Figure 1.9 (a), the shots are focused so as to reach the bull’s
eye (midpoint), but the arrows have reached only around this point. Hence the
shots are not accurate and also not precise.
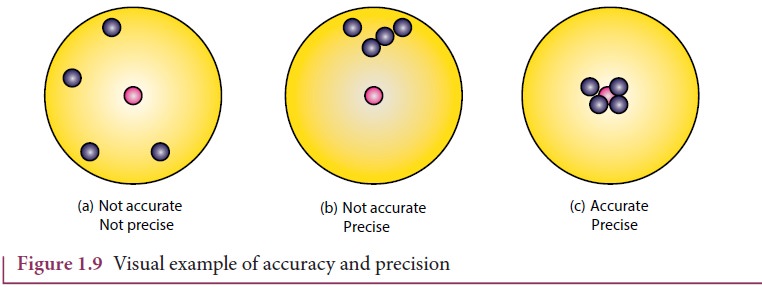
In
Figure 1.9 (b), all the shots are close to each other but not at the central
point. Hence the shots are said to be precise but not accurate. In Figure 1.9
(c), the shots are closer and also at the central point. Hence the shots are
both precise and accurate.
A numerical example
The
true value of a certain length is near 5.678 cm. In one experiment, using a
measuring instrument of resolution 0.1 cm, the measured value is found to be
5.5 cm. In another experiment using a measuring instrument of greater
resolution, say 0.01 cm, the length is found to be 5.38 cm. We find that the
first measurement is more accurate as it is closer to the true value, but it
has lesser precision. On the contrary, the second measurement is less accurate,
but it is more precise.
Errors in Measurement
The
uncertainty in a measurement is called an error. Random error, systematic error
and gross error are the three possible errors.
i) Systematic errors
Systematic
errors are reproducible inaccuracies that are consistently in the same
direction. These occur often due to a problem that persists throughout the
experiment. Systematic errors can be classified as follows
a. Instrumental errors
When
an instrument is not calibrated properly at the time of manufacture,
instrumental errors may arise. If a measurement is made with a meter scale
whose end is worn out, the result obtained will have errors. These errors can
be corrected by choosing the instrument carefully.
b. Imperfections in
experimental technique or procedure
These
errors arise due to the limitations in the experimental arrangement. As an
example, while performing experiments with a calorimeter, if there is no proper
insulation, there will be radiation losses. This results in errors and to
overcome these, necessary correction has to be applied.
c. Personal errors
These
errors are due to individuals performing the experiment, may be due to
incorrect initial setting up of the experiment or carelessness of the
individual making the observation due to improper precautions.
d. Errors due to external causes
The
change in the external conditions during an experiment can cause error in
measurement. For example, changes in temperature, humidity, or pressure during
measurements may affect the result of the measurement.
e. Least count error
Least
count is the smallest value that can be measured by the measuring instrument,
and the error due to this measurement is least count error. The instrument’s
resolution hence is the cause of this error. Least count error can be reduced
by using a high precision instrument for the measurement.
ii. Random errors
Random
errors may arise due to random and unpredictable variations in experimental conditions
like pressure, temperature, voltage supply etc. Errors may also be due to
personal errors by the observer who performs the experiment. Random errors are
sometimes called “chance error”. When
different readings are obtained by a
person every time he repeats the experiment, personal error occurs. For
example, consider the case of the thickness of a wire measured using a screw
gauge. The readings taken may be different for different trials. In this case,
a large number of measurements are made and then the arithmetic mean is taken.
If
n number of trial readings are taken in an experiment, and the readings are a1,
a2, a3,…………………. an. The arithmetic mean is
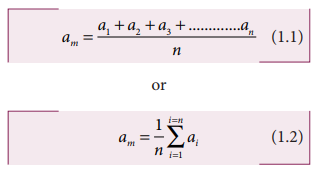
Usually this arithmetic mean is taken as the best possible true value of the quantity.
Certain
procedures to be followed to minimize experimental errors, along with examples
are shown in Table 1.8.
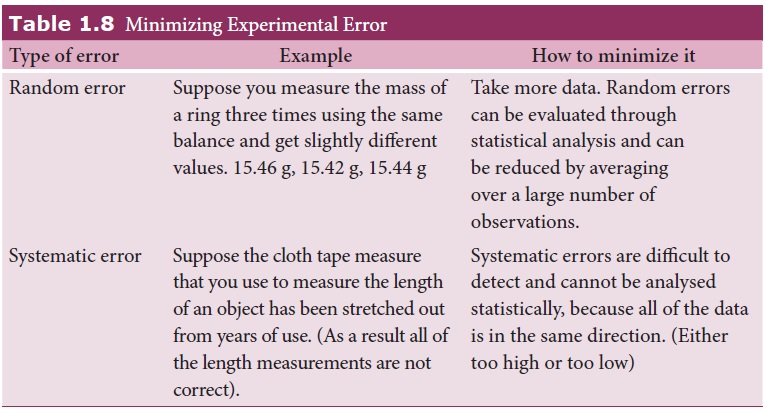
iii. Gross Error
The
error caused due to the shear carelessness of an observer is called gross
error.
For
example
i.
Reading an instrument without setting it properly.
ii.
Taking observations in a wrong manner without bothering about the sources of
errors and the precautions.
iii.
Recording wrong observations.
iv.
Using wrong values of the observations in calculations.
These errors can be minimized only when an observer is careful and mentally alert.
Error Analysis
i. Absolute Error
The
difference between the true value and the measured value of a quantity is
called absolute error. If a1, a2, a3, ………. an are the measured values of any quantity ‘a’ in an experiment performed n
times, then the arithmetic mean of these
values is called the true value (am)
of the quantity.
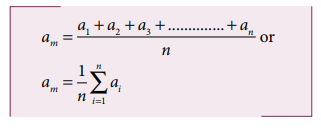
The
absolute error in measured values is given by
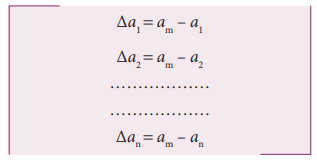
ii. Mean Absolute error
The
arithmetic mean of the magnitude of absolute errors in all the measurements is
called the mean absolute error.
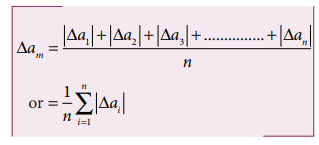
If
am is the true value and ∆am is the mean absolute error then the
magnitude of the quantity may lie between am
+ ∆am and am - ∆am
iii. Relative error
The
ratio of the mean absolute error to the mean value is called relative error.
This is also called as fractional error (or) relative error.

Thus Relative
error expresses how large the absolute error is compared to the total size of
the object measured. For example, a driver’s speedometer shows that his car is
travelling at 60 km h-1 when it is actually moving at 62 kmh-1.
Then absolute error of speedometer is 62-60 km h-1 = 2 km h-1
Relative error of the measurement is 2 km h-1 / 60 km h-1
= 0.033.
iv. Percentage error
The
relative error expressed as a percentage is called percentage error.

A percentage error very close to zero means one is close to the targeted value, which is good and acceptable. It is always necessary to understand whether error is due to impression of equipment used or a mistake in the experimentation.
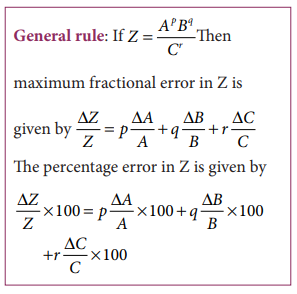
Propagation of errors
A
number of measured quantities may be involved in the final calculation of an
experiment. Different types of instruments might have been used for taking
readings. Then we may have to look at the errors in measuring various quantities,
collectively.
The
error in the final result depends on
i.
The errors in the individual measurements
ii.
On the nature of mathematical operations performed to get the final result. So
we should know the rules to combine the errors.
The
various possibilities of the propagation or combination of errors in different
mathematical operations are discussed below:
i. Error in the sum of two quantities
Let
∆A and ∆B be the absolute errors in the two quantities A and B respectively.
Then,
Measured
value of A = A ± ∆A
Measured
value of B = B ± ∆B
Consider
the sum, Z = A + B
The
error ∆Z in Z is then given by
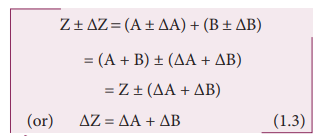
ii. Error in the difference of two quantities
Let ΔA and ΔB be the absolute
errors in the two quantities, A and B, respectively. Then,
Measured value of A = A ± ΔA
Measured value of B = B ± ΔB
Consider the difference, Z = A – B
The error ΔZ in Z is then given by
Z ± ΔZ = (A ± ΔA) – (B ± ΔB)
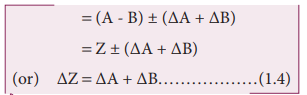
(iii) Error in the product of two quantities
Let ΔA and ΔB be the absolute
errors in the two quantities A, and B, respectively. Consider the product Z =
AB
The error ΔZ in Z is given by Z ±
ΔZ = (A ± ΔA) (B ± ΔB)
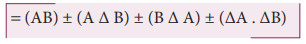
Dividing L.H.S by Z and R.H.S by
AB, we get,
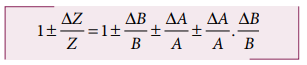
As ΔA /A, ΔB / B are
both small quantities, their product term
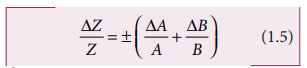
(iv) Error in the division or quotient of two quantities
Let ΔA and ΔB be the
absolute errors in the two quantities A and B respectively.
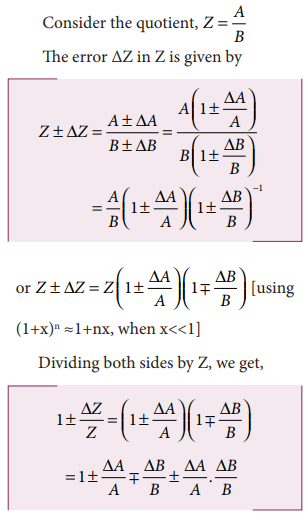
As the terms ΔA/A and
ΔB/B are small, their product term can be neglected.
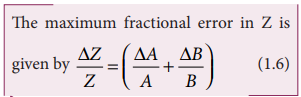
(v) Error in the power of a quantity
Consider the nth
power of A, Z = An The
error ΔZ in Z is given by
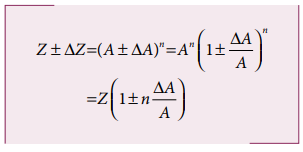
We get [(1+x)n
≈1+nx, when x<<1] neglecting remaining terms, Dividing both sides by Z
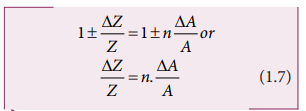
Related Topics