Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Theory Of Electrolytic Conductance
THEORY OF ELECTROLYTIC
CONDUCTANCE
Arrhenius theory of electrolytic conductance is
also known as Arrhenius theory of ionisation since electrolytic dissociation
into ions is considered here.
Postulates of Arrhenius Theory :
1. When dissolved in water, neutral electrolyte molecules are split up
into two types of charged particles.
These particles were called ions and the process was termed ionisation. The positively charged
particles were called cations and
those having negative charge were called anions.
The theory assumes that the ions are already
present in the solid electrolyte and these are held together by electrostatic
force. When placed in water, these neutral molecules dissociate to form separate anions and cations.
A+ B- -- -- > A+ + B-
For that reason, this theory may be referred to
as the theory of electrolytic dissociations.
2. The ions present in solution constantly
reunite to form neutral molecules. Thus there is a state of equilibrium between
the undissociated molecules and the ions.
AB < -- --- > A+ + B-
Applying the Law of Mass Action to the ionic
equilibrium we have,
[ A + ][ B - ] / [AB] = K
where K is called the Dissociation constant.
3.The charged ions are free to move through the
solution to the oppositely charged electrode. This is called as migration of
ions. This movement of the ions constitutes the electric current through
electrolytes. This explains the conductivity of electrolytes as well as the
phenomenon of electrolysis.
4.The electrical conductivity of an electrolyte
solution depends on the number of ions present in solution. Thus the degree of
dissociation of an electrolyte determines whether it is a strong electrolyte or
a weak electrolyte.
We know that electrolytes dissociate in
solution to form positive ions (cations) and negative ions (anions).
AgNO3 -- -- -
> Ag+ + NO3-
CuSO -- -- -
> Cu2+ + SO 2-
H2SO-- -- - > 2H+ + SO 2-
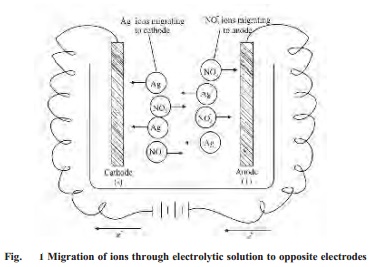
As the current is passed between the electrode
of the electrolytic cell, the ions migrate to the opposite electrodes. Thus in
the electrolytic solution of AgNO3, the cations (Ag+)
will move to the cathode and anions (NO3- ) will move to
the anode. Usually different ions move with different rates. The migration of
ions through the electrolytic solution can be demonstrated by the following
experiments.
5.The
properties of solution of electrolytes are the properties of ions. The solution
of electrolyte as a whole is electrically neutral unless an electric
field is applied to the electrodes dipped into
it. Presence of hydrogen ions (H+) renders the solution acidic while
presence of hydroxide ions (OH- ) renders the solution basic.
6.There are two types of electrolytes. Strong electrolytes
are those when dissolved in water are completely dissociated (ionised) into
ions of positive and negative charges.
The total number of cations and anions produced are equal to those in the
formula of the electrolyte.
Al2(SO4)3 --
> 2Al3+ + 3SO 2-
![]() NaCl,
KCl, AgNO3 etc., are few examples of strong electrolytes.
NaCl,
KCl, AgNO3 etc., are few examples of strong electrolytes.
In the case of weak electrolytes, there is
partial dissociation into ions in water and an equilibrium exists between the
dissociated ions and the undissociated electrolyte.
(e.g.,) CH3COOH < -- --- > CH3COO- + H+. Acetic acid is a weak
electrolyte in water and unionised acetic acid
molecules are in equilibrium with the acetate anions and H+ ions in
solution.
Evidences of Arrhenius theory
of electrolytic dissociation
1. The enthalpy of neutralisation of strong
acid by strong base is a constant value and is equal to -57.32 kJ. gm.equiv -1.
This aspect is well explained by adopting Arrhenius theory of electrolytic
dissociation. Strong acids and strong bases are completely ionised in water and
produce H+ and OH- ions respectively along with the
counter ions. The net reaction in the acid-base neutralisation is the formation
of water from H+ and OH- ions.
H+ + OH- -- -- - > H2O, DHro = -57.32
kJ.mol -1
2.The
colour of certain salts or their solution is due to the ions present. For
example, copper sulphate is blue due to Cu2+ ions. Nickel salts are
green due to Ni2+ ions. Metallic chromates are yellow due to CrO42-
ions.
3.Ostwalds
dilution law, common ion effect and solubility product and other such concepts
are based on Arrhenius theory.
4.Chemical reactions between electrolytes are
almost ionic reactions. This is because these are essentially the reaction
between oppositely charged ions. For example,
Ag+ + Cl- --- -- > AgCl
¯
5.Electrolytic solutions conduct
current due to the presence of ions which migrate in the presence of electric
field.
6.Colligative properties depend on the number
of particles present in the solution. Electrolytic solution has abnormal
colligative properties. For example, 0.1 molal solution of NaCl has elevation
of boiling point about twice that of 0.1 molal solution of non-electrolyte. The
abnormal colligative properties of electrolytic solutions can be explained with
theory of electrolytic dissociation.
Ostwald's dilution law for
weak electrolytes
According to Arrhenius theory, weak
electrolytes partially dissociate into ions in water which are in equilibrium
with the undissociated electrolyte molecules. Ostwald's dilution law relates
the dissociation constant of the weak electrolyte with the degree of
dissociation and the concentration of the weak electrolyte. Consider the
dissociation equilibrium of CH3COOH which is a weak electrolyte in
water.
CH3COOH <-- -- > CH3COO- + H+
Ka
= [ H + ][CH 3COO
- ] / [CH3COOH]
a is the
degree of dissociation which represents the fraction of total concentration
of CH3COOH that exists in the completely ionised state. Hence (1 - a) is the
fraction of the total concentration of CH3COOH, that exists in the
unionised state. If 'C' is the total concentration of CH 3COOH
initially, then at equilibrium Ca, Ca and C (1 - a) represent the concentration of H+,
CH3COO- and CH3COOH respectively.
Then Ka
= (Ca .C a) / C (1-a ) / a2 C / (1-a)
If a is too small,
then Ka = a2C
a = root(Ka/C)
also [H+] = [CH3COO-]
= Ca
[H+] = root(Ka.C)
Ka=
a2C / (1-a) is known as the Ostwalds dilution law. For weak bases,
Kb=
a2C / (1-a) and a = rt(Kb/C) at a = small values.
Kb = dissociation constant for weak base.
This law fails for strong electrolytes. For
strong electrolytes, a tends to 1.0 and therefore Ka
increases tremendously.
Related Topics