Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
The Wave Nature Of Electrons
THE WAVE NATURE OF ELECTRONS
It has been made clear that, if a substance is divided into finer and
finer pieces, we reach molecules and atoms, then we realize that the atoms
consist of electrons and nuclei. It has been clarified that matter is a
collection of ultra microscopic particles. Upto the 19th century, these
particles were considered to move obeying Newtonian mechanics and Maxwellian
electromagnetism. However, this view point has became doubtful after the
proposal of the Bohr model of the atomic structure (Bohr's
quantum theory).
On the
other hand, light had been
considered to be electromagnetic waves. However, after the discovery of light quanta (photons), it was clarified that the
light has wave nature at one time and particle nature at another time. Therefore,
light has a kind of duality.
The idea of deBroglie wave nature waves or deBroglie matter waves is
based on the fact that light has both wave and particle nature. Hence particle
like electron or proton can also be considered to be 'particle' with 'wave nature'.
Einstein's
relations which connect the particle and wave aspects in light quanta
E = hν, p = h / ë
would be
satisfied for de Broglie matter waves as well. Therefore the relations, Eq.(1),
are often called Einstein-de Broglie's
relations.
If we
apply these relations to the case of the Bohr model of the hydrogen atom, we
can well understand its possibility as follows. If we consider that the
electron in a hydrogen atom moves at constant speed along a circular orbit
around the nucleus (proton), the quantum
condition in Bohr's quantum theory is written as Eq(2). By using Einstein's
relation p = h/λ in this
equation, the quantum condition is written
2πa = nλ, (n = 1, 2, 3, ....) (2)
This
equation means that the circumference of the circular orbit of the electron
must be a integral multiple of the wavelength of de Broglie wave. In other
word, de-Broglie wave accompanying the motion of the electron should be
continuous. Therefore, we can easily understand the quantum condition that determines
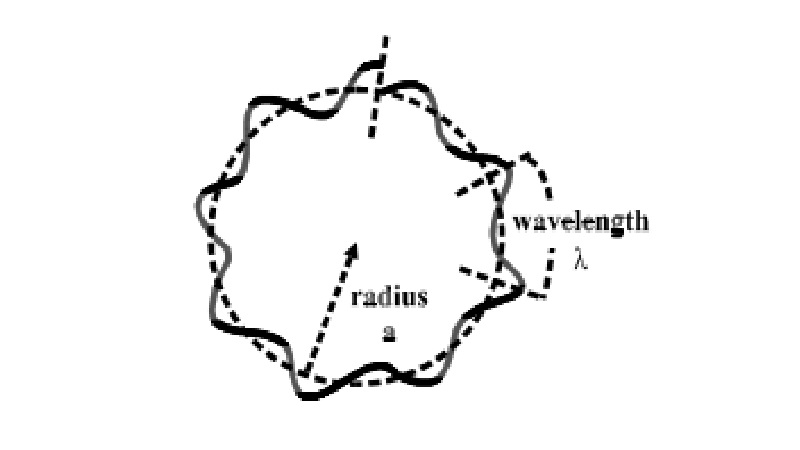
the stationary states by considering the continuity of de Broglie waves(fig).
Bohr's
quantum condition. The condition for stationary states
The circumference of the circular orbit
of the electron should be an integral multiple of the wavelength of de Broglie
wave, otherwise the wave cannot be smoothly continuous.
Energy of electron in
an atom. By applying Schrodinger wave equation to hydrogen atom, the energy of
electron (En) was found as :
En = -2ð2me4 / h2 n2
where n is the principal quantum number.
This expression is same as Bohr's equation for energy of electron in a hydrogen
atom.
Substituting the values of m, e and h in
relation (1), we get
En = -
1312 / n2 kJ mol−1
Significance of negative electronic energy
Related Topics