Chapter: Basic & Clinical Pharmacology : The Gonadal Hormones & Inhibitors
The Progestins
THE PROGESTINS
Natural Progestins: Progesterone
Progesterone is the
most important progestin in humans. In addition to having important hormonal
effects, it serves as a precursor to the estrogens, androgens, and
adrenocortical steroids. It is synthesized in the ovary, testis, and adrenal
cortex from circulating cholesterol. Large amounts are also synthesized and
released by the placenta during pregnancy.
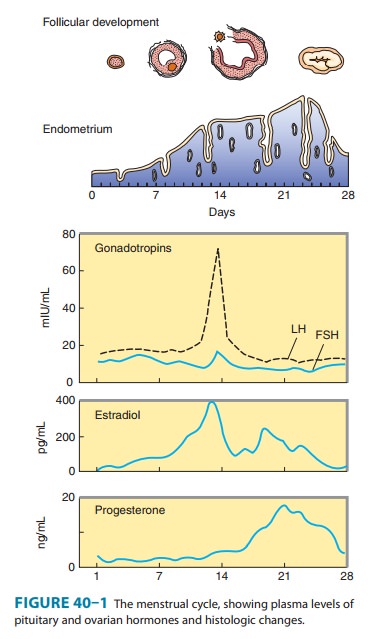
In the ovary,
progesterone is produced primarily by the corpus luteum. Normal males appear to
secrete 1–5 mg of progesterone daily, resulting in plasma levels of about 0.03
mcg/dL. The level is only slightly higher in the female during the follicular
phase of the cycle, when only a few milligrams per day of progesterone are
secreted. During the luteal phase, plasma levels range from 0.5 mcg/dL to more
than 2 mcg/dL (Figure 40–1). Plasma levels of progesterone are further elevated
and reach their peak levels in the third trimester of pregnancy.
Synthetic Progestins
A variety of progestational compounds have
been synthesized. Some are active when given by mouth. They are not a uniform
group of compounds, and all of them differ from progesterone in one or more
respects. Table 40–2 lists some of these compounds and their effects. In
general, the 21-carbon compounds (hydroxy-progesterone, medroxyprogesterone,
megestrol, and dimethisterone) are the most closely related, pharmacologically
as well as chemi-cally, to progesterone. A new group of third-generation
synthetic progestins has been introduced, principally as components of oral
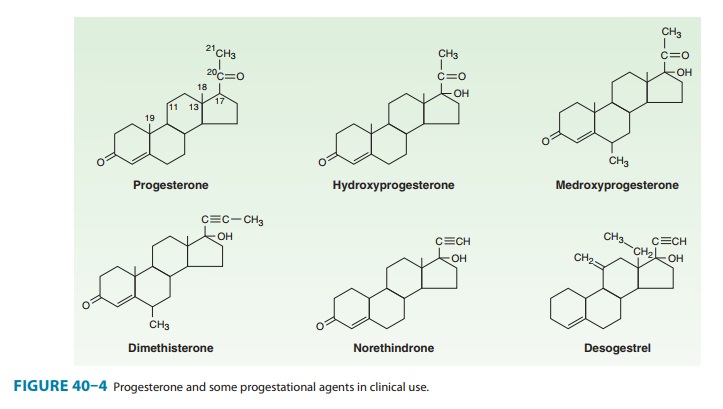
contraceptives. These “19-nor, 13-ethyl” steroid compounds
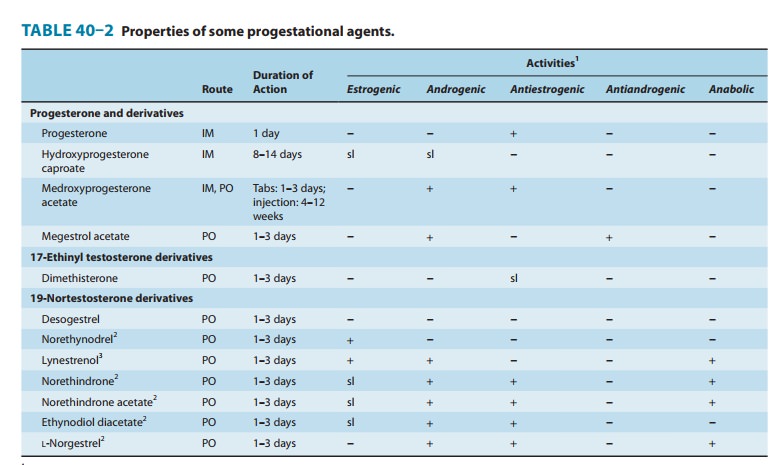
include desogestrel (Figure 40–4), gestodene,
and norgestimate. They are claimed to have lower androgenic activity than older
synthetic progestins.
Pharmacokinetics
Progesterone is
rapidly absorbed following administration by any route. Its half-life in the
plasma is approximately 5 minutes, and small amounts are stored temporarily in
body fat. It is almost completely metabolized in one passage through the liver,
and for that reason it is quite ineffective when the usual formulation is
administered orally. However, high-dose oral micronized proges-terone preparations
have been developed that provide adequate progestational effect.
In the liver,
progesterone is metabolized to pregnanediol and conjugated with glucuronic
acid. It is excreted into the urine as pregnanediol glucuronide. The amount of
pregnanediol in the urine has been used as an index of progesterone secretion.
This measure has been very useful despite the fact that the proportion of
secreted progesterone converted to this compound varies from day to day and
from individual to individual. In addition to pro-gesterone, 20α- and 20β-hydroxyprogesterone
(20α-
and 20β-hydroxy-4-pregnene-3-one)
are also found. These compounds have about one fifth the progestational
activity of progesterone in humans and other species. Little is known of their
physiologicrole, but 20α-hydroxyprogesterone is produced in large
amounts in some species and may be of some importance biologically.The usual
routes of administration and durations of action of the synthetic progestins
are listed in Table 40–2. Most of these agents are extensively metabolized to
inactive products that are excreted mainly in the urine.
Physiologic Effects
A. Mechanism
The mechanism of action of progesterone—described in more detail above—is similar to that of other steroid hormones. Progestins enter the cell and bind to progesterone receptors that are distributed between the nucleus and the cytoplasm. The ligand-receptor complex binds to a progesterone response element (PRE) to activate gene transcription. The response element for progester-one appears to be similar to the corticosteroid response element, and the specificity of the response depends upon which receptor is present in the cell as well as upon other cell-specific receptor coregulators and interacting transcription factors. The progester-one-receptor complex forms a dimer before binding to DNA. Like the estrogen receptor, it can form heterodimers as well as homodi-mers between two isoforms, A and B. These isoforms are produced by alternative splicing of the same gene.
B. Effects of Progesterone
Progesterone has
little effect on protein metabolism. It stimulates lipoprotein lipase activity
and seems to favor fat deposition. The effects on carbohydrate metabolism are
more marked. Progesterone increases basal insulin levels and the insulin
response to glucose. There is usually no manifest change in carbohydrate
tolerance. In the liver, progesterone promotes glycogen storage, possibly by
facilitating the effect of insulin. Progesterone also promotes ketogenesis.
Progesterone
can compete with aldosterone for the mineralo-corticoid receptor of the renal
tubule, causing a decrease in Na+
reabsorption. This leads to an increased secretion of aldosterone by the
adrenal cortex (eg, in pregnancy). Progesterone increases body temperature in
humans. The mechanism of this effect is not known, but an alteration of the
temperature-regulating centers in the hypothalamus has been suggested.
Progesterone also alters the function of the respiratory centers. The
ventilatory response to CO2 is
increased by progesterone but synthetic progestins with an ethinyl group do not
have respiratory effects. This leads to a mea-surable reduction in arterial and
alveolar PCO2
during pregnancy and in the luteal phase of the menstrual cycle. Progesterone
and related steroids also have depressant and hypnotic effects on the brain.
Progesterone is
responsible for the alveolobular development of the secretory apparatus in the
breast. It also participates in the preovulatory LH surge and causes the
maturation and secretory changes in the endometrium that are seen following
ovulation (Figure 40–1).
Progesterone decreases
the plasma levels of many amino acids and leads to increased urinary nitrogen
excretion. It induces changes in the structure and function of smooth
endoplasmic reticulum in experimental animals. Other effects of progesterone
and its analogs are noted below in the section, Hormonal Contraception.
C. Synthetic Progestins
The 21-carbon
progesterone analogs antagonize aldosterone-induced sodium retention (see
above). The remaining compounds (“19-nortestosterone” third-generation agents)
produce a decidual change in the endometrial stroma, do not support pregnancy
in test animals, are more effective gonadotropin inhibitors, and may have
minimal estrogenic and androgenic or anabolic activity (Table 40–2; Figure
40–4). They are sometimes referred to as “impeded androgens.” Progestins
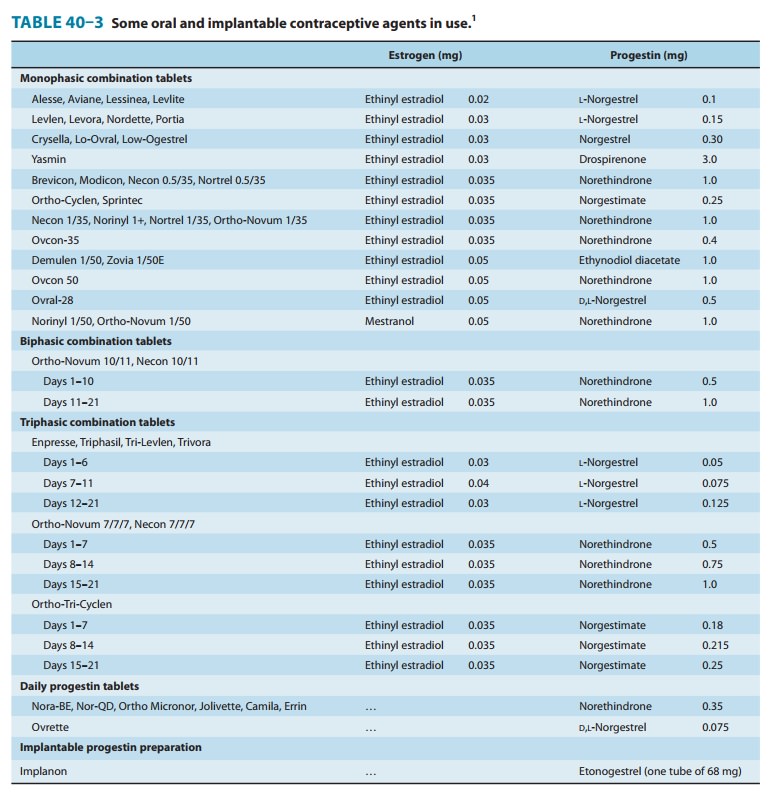
without androgenic activity include desogestrel, norgestimate, and gestodene.
The first two of these compounds are dispensed in combination with ethinyl
estra-diol for oral contraception (Table 40–3) in the USA. Oral contra-ceptives
containing the progestins cyproterone acetate (also an antiandrogen) in
combination with ethinyl estradiol are investiga-tional in the USA.
Clinical Uses
A. Therapeutic Applications
The major uses of progestational hormones are for hormone replacement therapy (see above) and hormonal contraception . In addition, they are useful in producing long-term ovarian suppression for other purposes. When used alone in large doses parenterally (eg, medroxyprogesterone acetate, 150 mg intramus-cularly every 90 days), prolonged anovulation and amenorrhea result. This therapy has been employed in the treatment of dys-menorrhea, endometriosis, and bleeding disorders when estrogens are contraindicated, and for contraception.
The major problem with this regimen is
the prolonged time required in some patients for ovulatory function to return
after cessation of therapy. It should not be used for patients planning a
pregnancy in the near future. Similar regimens will relieve hot flushes in some
menopausal women and can be used if estrogen therapy is contraindicated.
Medroxyprogesterone
acetate, 10–20 mg orally twice weekly—or intramuscularly in doses of 100 mg/m2 every 1–2 weeks—will
prevent menstruation, but it will not arrest accelerated bone maturation in
children with precocious puberty.Progestins do not appear to have any place in
the therapy of threatened or habitual abortion. Early reports of the usefulness
of these agents resulted from the unwarranted assumption that after several
abortions the likelihood of repeated abortions was over 90%. When
progestational agents were administered to patients with previous abortions, a
salvage rate of 80% was achieved. It is now recognized that similar patients
abort only 20% of the time even when untreated. On the other hand, progesterone
was given experimentally to delay premature labor with encouraging results.
Progesterone
and medroxyprogesterone have been used in the treatment of women who have
difficulty in conceiving and who demonstrate a slow rise in basal body
temperature. There is no convincing evidence that this treatment is effective.
Preparations
of progesterone and medroxyprogesterone have been used to treat premenstrual
syndrome. Controlled studies have not confirmed the effectiveness of such
therapy except when doses sufficient to suppress ovulation have been used.
B. Diagnostic Uses
Progesterone
can be used as a test of estrogen secretion. The administration of
progesterone, 150 mg/d, or medroxyprogester-one, 10 mg/d, for 5–7 days, is
followed by withdrawal bleeding in amenorrheic patients only when the
endometrium has been stimulated by estrogens. A combination of estrogen and
progestin can be given to test the responsiveness of the endometrium in
patients with amenorrhea.
Contraindications, Cautions, & Adverse Effects
Studies
of progestational compounds alone and with combination oral contraceptives
indicate that the progestin in these agents may increase blood pressure in some
patients. The more androgenic progestins also reduce plasma HDL levels in
women. (See Hormonal Contraception, below.) Two recent studies suggest that
combined progestin plus estrogen replacement therapy in postmenopausal women
may increase breast cancer risk significantly compared with the risk in women
taking estrogen alone. These findings require careful examination and if
confirmed will lead to important changes in postmenopausal hormone replacement
practice.
Related Topics