Chapter: Basic & Clinical Pharmacology : The Gonadal Hormones & Inhibitors
Adverse Effects - Hormonal Contraception
Adverse Effects
The incidence of
serious known toxicities associated with the use of these drugs is low—far
lower than the risks associated with pregnancy. There are a number of
reversible changes in intermedi-ary metabolism. Minor adverse effects are
frequent, but most are mild and many are transient. Continuing problems may
respond to simple changes in pill formulation. Although it is not often
necessary to discontinue medication for these reasons, as many as one third of
all patients started on oral contraception discontinue use for reasons other than
a desire to become pregnant.
A. Mild Adverse Effects
1. Nausea,mastalgia,
breakthrough bleeding, and edema are related to the amount of estrogen in the
preparation. These effects can often be alleviated by a shift to a preparation
con-taining smaller amounts of estrogen or to agents containing progestins with
more androgenic effects.
2. Changes in serum
proteins and other effects on endocrine function (see above) must be taken into
account when thyroid, adrenal, or pituitary function is being evaluated.
Increases in sedimentation rate are thought to be due to increased levels of
fibrinogen.
3. Headache
is mild and often transient. However, migraine is often made worse and has been
reported to be associated with an increased frequency of cerebrovascular accidents.
When this occurs or when migraine has its onset during therapy with these
agents, treatment should be discontinued.
4. Withdrawal bleeding
sometimes fails to occur—most often with combination preparations—and may cause
confusion with regard to pregnancy. If this is disturbing to the patient, a
different preparation may be tried or other methods of contra-ception used.
B. Moderate Adverse Effects
Any of the following
may require discontinuance of oral contra-ceptives:
1.
Breakthrough bleeding is the most common problem in using progestational agents
alone for contraception. It occurs in as many as 25% of patients. It is more
frequently encountered in patients taking low-dose preparations than in those
taking combination pills with higher levels of progestin and estrogen. The
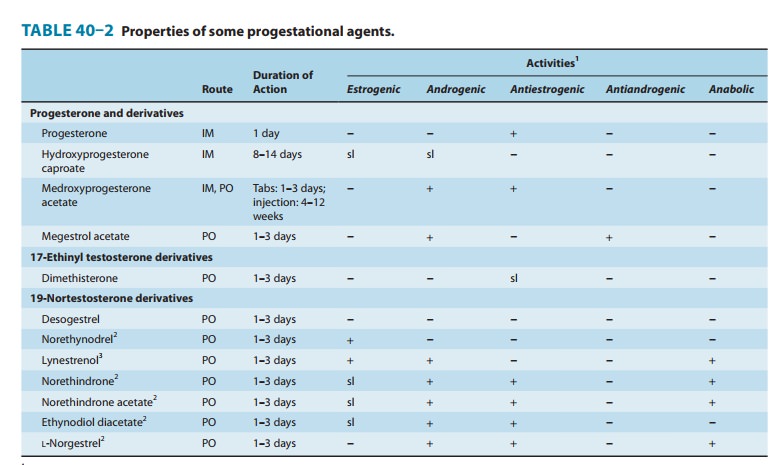
biphasic and triphasic oral contraceptives (Table 40–3) decrease breakthrough
bleeding without increasing the total hormone content.
2. Weight gain is more
common with the combination agents containing androgen-like progestins. It can
usually be con-trolled by shifting to preparations with less progestin effect
or by dieting.
3.
Increased skin pigmentation may occur, especially in dark-skinned women. It
tends to increase with time, the incidence being about 5% at the end of the first
year and about 40% after 8 years. It is thought to be exacerbated by vitamin B
deficiency. It is often reversible upon discontinuance of medication but may
disappear very slowly.
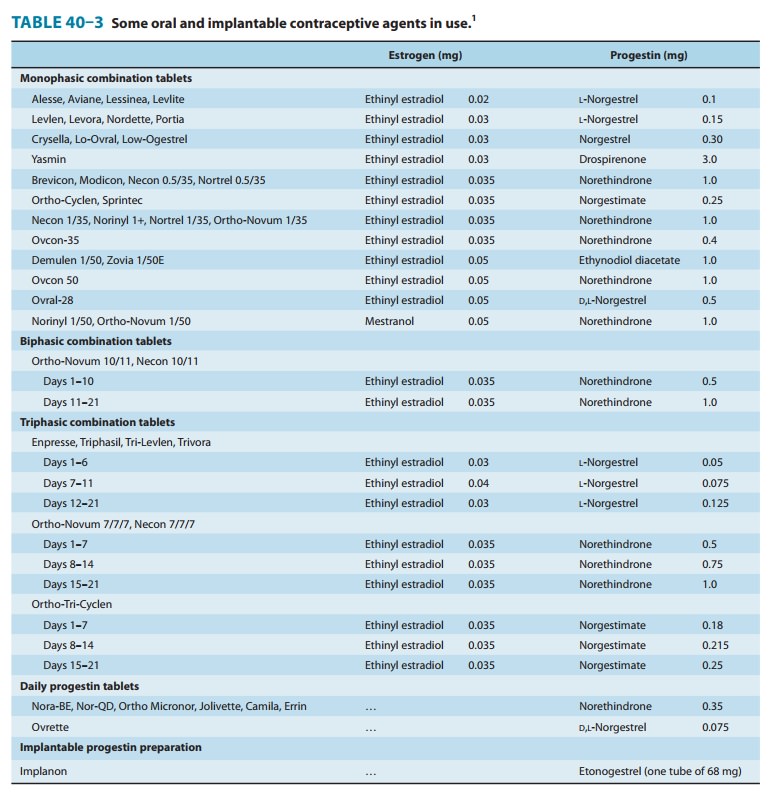
4. Acne may be
exacerbated by agents containing androgen-like progestins (Table 40–2), whereas
agents containing large amounts of estrogen usually cause marked improvement in
acne.
5. Hirsutism may also
be aggravated by the “19-nortestosterone” derivatives, and combinations
containing nonandrogenic progestins are preferred in these patients.
6. Ureteral dilation
similar to that observed in pregnancy has been reported, and bacteriuria is
more frequent.
7. Vaginal infections
are more common and more difficult to treat in patients who are using oral
contraceptives.
8. Amenorrhea occurs
in some patients. Following cessation of administration of oral contraceptives,
95% of patients with normal menstrual histories resume normal periods and all
but a few resume normal cycles during the next few months. How-ever, some
patients remain amenorrheic for several years. Many of these patients also have
galactorrhea. Patients who have had menstrual irregularities before taking oral
contraceptives are particularly susceptible to prolonged amenorrhea when the
agents are discontinued. Prolactin levels should be measured in these patients,
since many have prolactinomas.
C. Severe Adverse Effects
1. Vascular disorders—Thromboembolism was
one of theearliest of the serious unanticipated effects to be reported and has
been the most thoroughly studied.
a. Venous thromboembolic disease—Superficial or deep throm-boembolic disease
in women not taking oral contraceptives occurs in about 1 patient per 1000
woman years. The overall incidence of these disorders in patients taking
low-dose oral contraceptives is about threefold higher. The risk for this
disorder is increased during the first month of contraceptive use and remains
constant for several years or more. The risk returns to normal within a month
when use is discontinued. The risk of venous thrombosis or pulmonary embolism
is increased among women with predis-posing conditions such as stasis, altered
clotting factors such asantithrombin III, increased levels of homocysteine, or
injury. Genetic disorders, including mutations in the genes governing the production
of protein C (factor V Leiden), protein S, hepatic cofactor II, and others,
markedly increase the risk of venous thromboembolism. The incidence of these
disorders is too low for cost-effective screening by current methods, but prior
episodes or a family history may be helpful in identifying patients with
increased risk.
The incidence of
venous thromboembolism appears to be related to the estrogen but not the
progestin content of oral con-traceptives and is not related to age, parity,
mild obesity, or ciga-rette smoking. Decreased venous blood flow, endothelial
proliferation in veins and arteries, and increased coagulability of blood
resulting from changes in platelet functions and fibrinolytic systems
contribute to the increased incidence of thrombosis. The major plasma inhibitor
of thrombin, antithrombin III, is substan-tially decreased during oral
contraceptive use. This change occurs in the first month of treatment and lasts
as long as treatment per-sists, reversing within a month thereafter.
b. Myocardial infarction—The
use of oral contraceptives isassociated with a slightly higher risk of
myocardial infarction in women who are obese, have a history of preeclampsia or
hyper-tension, or have hyperlipoproteinemia or diabetes. There is a much higher
risk in women who smoke. The risk attributable to oral contraceptives in women
30–40 years of age who do not smoke is about 4 cases per 100,000 users per
year, as compared with 185 cases per 100,000 among women 40–44 who smoke
heavily. The association with myocardial infarction is thought to involve
acceleration of atherogenesis because of decreased glucose tolerance, decreased
levels of HDL, increased levels of LDL, and increased platelet aggregation. In
addition, facilitation of coronary arterial spasm may play a role in some of
these patients. The progestational component of oral contraceptives decreases
HDL cholesterol levels, in proportion to the androgenic activity of the
progestin. The net effect, therefore, will depend on the specific composition
of the pill used and the patient’s susceptibility to the particular effects.
Recent studies suggest that risk of infarc-tion is not increased in past users
who have discontinued oral contraceptives.
c. Cerebrovascular disease—The
risk of stroke is concentratedin women over age 35. It is increased in current
users of oral con-traceptives but not in past users. However, subarachnoid
hemor-rhages have been found to be increased among both current and past users
and may increase with time. The risk of thrombotic or hemorrhagic stroke
attributable to oral contraceptives (based on older, higher-dose preparations)
has been estimated to about 37 cases per 100,000 users per year.
In summary, available
data indicate that oral contraceptives increase the risk of various cardiovascular
disorders at all ages and among both smokers and nonsmokers. However, this risk
appears to be concentrated in women 35 years of age or older who are heavy
smokers. It is clear that these risk factors must be considered in each
individual patient for whom oral contraceptives are being considered. Some
experts have suggested that screening for coagu-lopathy should be performed
before starting oral contraception.
2. Gastrointestinal disorders—Many cases of
cholestaticjaundice have been reported in patients taking progestin-containing
drugs. The differences in incidence of these disorders from one population to
another suggest that genetic factors may be involved. The jaundice caused by
these agents is similar to that produced by other 17-alkyl-substituted
steroids. It is most often observed in the first three cycles and is
particularly common in women with a his-tory of cholestatic jaundice during
pregnancy. Jaundice and pruri-tus disappear 1–8 weeks after the drug is
discontinued.
These
agents have also been found to increase the incidence of symptomatic
gallbladder disease, including cholecystitis and cho-langitis. This is probably
the result of the alterations responsible for jaundice and bile acid changes
described above.
It also appears that
the incidence of hepatic adenomas is increased in women taking oral
contraceptives. Ischemic bowel disease secondary to thrombosis of the celiac
and superior and inferior mesenteric arteries and veins has also been reported
in women using these drugs.
3. Depression—Depression of
sufficient degree to require ces-sation of therapy occurs in about 6% of
patients treated with some preparations.
4. Cancer—The occurrence of
malignant tumors in patientstaking oral contraceptives has been studied
extensively. It is now clear that these compounds reduce the risk of endometrial and ovarian cancer. The lifetime
risk of breast cancer in the population as a whole does not seem to be affected
by oral contraceptive use. Some studies have shown an increased risk in younger
women, and it is possible that tumors that develop in younger women become
clinically apparent sooner. The relation of risk of cervical cancer to oral
contraceptive use is still controversial. It should be noted that a number of
recent studies associate the use of oral contraceptives by women who are
infected with human papillo-mavirus with an increased risk of cervical cancer.
5. Other—In
addition to the above effects, a number of otheradverse reactions have been
reported for which a causal relation has not been established. These include
alopecia, erythema multi-forme, erythema nodosum, and other skin disorders.
Related Topics