Chapter: Basic & Clinical Pharmacology : The Gonadal Hormones & Inhibitors
The Estrogens
THE ESTROGENS
Estrogenic
activity is shared by a large number of chemical substances. In addition to
the variety of steroidal estrogens derived from animal sources, numerous
nonsteroidal estrogens have been synthesized. Many phenols are estrogenic, and
estrogenic activity has been identified in such diverse forms of life as those
found in ocean sediments. Estrogen-mimetic compounds (flavonoids) are found in
many plants, including saw palmetto, and soybeans and other foods. Studies have
shown that a diet rich in these plant products may cause slight estrogenic
effects. Additionally, some compounds used in the manufacture of plastics
(bisphenols, alkylphenols, phthalate phenols) have been found to be
estro-genic. It has been proposed that these agents are associated with an
increased breast cancer incidence in both women and men in the industrialized
world.
Natural Estrogens
The major estrogens
produced by women are estradiol
(estradiol-17β,
E2), estrone (E1), and estriol
(E3) (Figure 40–2).
Estradiol is the major secretory product of the ovary. Although some estrone is
produced in the ovary, most estrone and estriol are formed in the liver
from estradiol or in peripheral tissues from androstenedione and other
androgens (see Figure 39–1). As noted above, duringthe first part of the
menstrual cycle estrogens are produced in the ovarian follicle by the theca and
granulosa cells. After ovulation, the estrogens as well as progesterone are
synthesized by the luteinized granulosa and theca cells of the corpus luteum,
and the pathways of biosynthesis are slightly different.
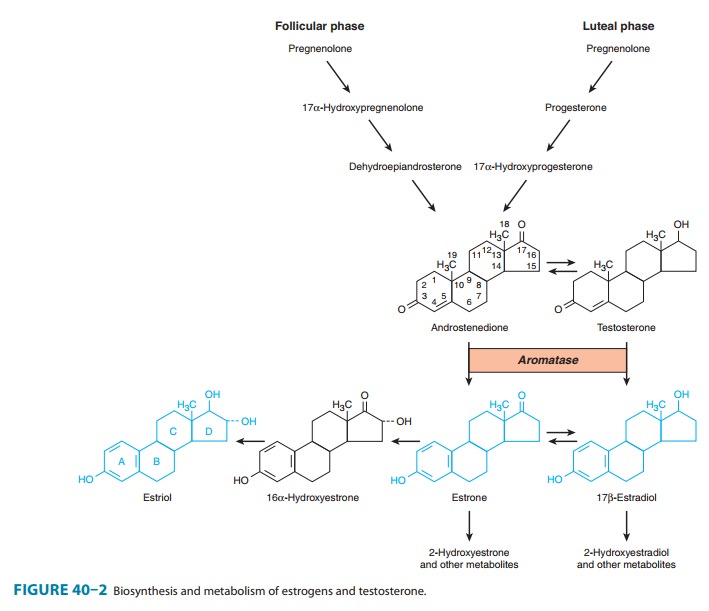
During pregnancy, a
large amount of estrogen is synthesized by the fetoplacental unit—consisting of
the fetal adrenal zone, secret-ing androgen precursor, and the placenta, which
aromatizes it into estrogen. The estriol synthesized by the fetoplacental unit
is released into the maternal circulation and excreted into the urine. Repeated
assay of maternal urinary estriol excretion has been used in the assessment of
fetal well-being.
One of the most
prolific natural sources of estrogenic substances is the stallion, which
liberates more of these hormones than the pregnant mare or pregnant woman. The
equine estrogens—equilenin and equilin—and their congeners are unsaturated in
the B as well as the A ring and are excreted in large quantities in urine, from
which they can be recovered and used for medicinal purposes.
In normal women, estradiol is produced at a rate that varies during the menstrual cycle, resulting in plasma levels as low as 50 pg/mL in the early follicular phase to as high as 350–850 pg/mL at the time of the preovulatory peak (Figure 40–1).
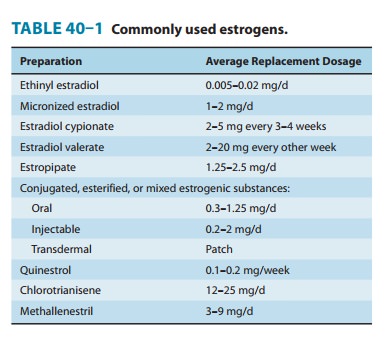
Synthetic Estrogens
A variety of chemical
alterations have been applied to the natural estrogens. The most important
effect of these alterations has been to increase their oral effectiveness. Some
structures are shown in Figure 40–3. Those with therapeutic use are listed in
Table 40–1.
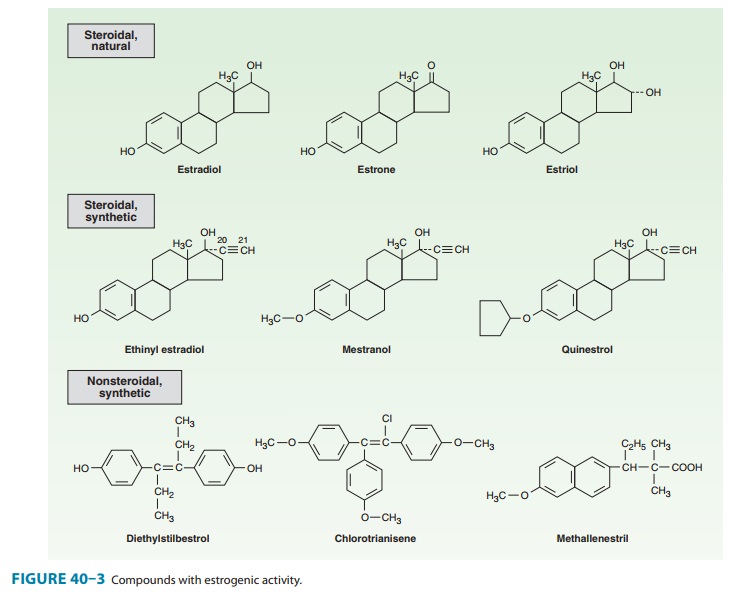
In addition to the
steroidal estrogens, a variety of nonsteroidal compounds with estrogenic
activity have been synthesized and used clinically. These include dienestrol,
diethylstilbestrol, benze-strol, hexestrol, methestrol, methallenestril, and
chlorotrianisene (Figure 40–3).
Pharmacokinetics
When
released into the circulation, estradiol binds strongly to an α2
globulin (sex hormone-binding globulin [SHBG]) and with lower affinity to
albumin. Bound estrogen is relatively unavailable for dif-fusion into cells,
and it is the free fraction that is physiologically active. Estradiol is
converted by the liver and other tissues to estrone and estriol (Figure 40–2)
and their 2-hydroxylated derivatives and conjugated metabolites (which are too
insoluble in lipid to cross the cell membrane readily) and excreted in the
bile. Estrone and estriol have low affinity for the estrogen receptor. However,
the conjugates may be hydrolyzed in the intestine to active, reabsorbable
com-pounds. Estrogens are also excreted in small amounts in the breast milk of
nursing mothers.
Because
significant amounts of estrogens and their active metabolites are excreted in
the bile and reabsorbed from the intes-tine, the resulting enterohepatic
circulation ensures that orally administered estrogens will have a high ratio
of hepatic to periph-eral effects. As noted below, the hepatic effects are
thought to be responsible for some undesirable actions such as synthesis of
increased clotting factors and plasma renin substrate. The hepatic effects of
estrogen can be minimized by routes that avoid first-pass liver exposure, ie,
vaginal, transdermal, or by injection.
Related Topics