Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Sublimation - Purification of Organic compounds
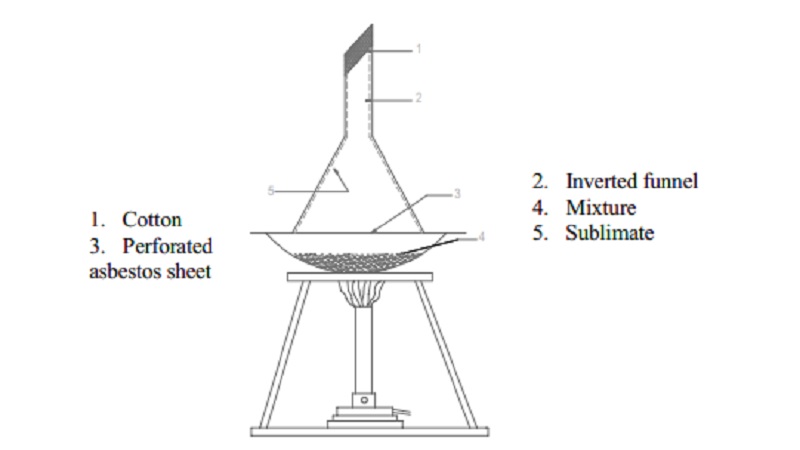
Sublimation
Certain
solid substances like Naphthalene or camphor when heated pass directly from
solid to the vapour state without melting. The vapours when cooled give back
the solid substance. This process is known as sublimation.
1. Cotton 2. Inverted
funnel
3. Perforated 4. Mixture
asbestos sheet 5. Sublimate
This process is very helpful in separating a volatile solid from a
non-volatile solid. The powdered substance is taken in a China dish and covered
with a perforated filter paper and an inverted funnel. The dish is carefully
heated on a sand bath.
The vapours passing through the holes in the paper condense on the inner
sides of the funnel. The non-volatile impurities remain in the dish.
Need for purification
of organic compounds
The
organic compounds obtained from natural sources are not pure. They contain a
number of other compounds which occur with them. Similarly, the organic
compounds prepared in the laboratory are also not pure. They are found to
contain other products formed during the reaction. In order to investigate the
structure and properties of an organic compound, it should be in the purest
form. Hence purification of organic compounds become essential.
Various methods used for purification and
separation of organic compounds are:
i)
Crystallisation
ii) Fractional Crystallisation
iii)
Sublimation
iv) Distillation
v)
Extraction with solvents
vi)
Chromatography
Related Topics