Chapter: Biochemistry: Biochemistry and the Organization of Cells
Predicting Reactions
Predicting Reactions
Let us
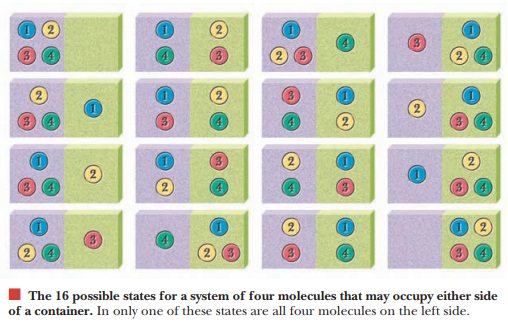
consider a very simple system to illustrate the concept of entropy. We place
four molecules in a con-tainer. There is an equal chance that each molecule
will be on the left or on the right side of the container. Mathematically
stated, the probability of finding a
given molecule on one side is 1/2. We can express any prob-ability as a
fraction ranging from 0 (impossible) to 1 (completely certain). We can see that
16 possible ways exist to arrange the four molecules in the container. In only
one of these will all four molecules lie on the left side, but six possible
arrangements exist with the four molecules evenly distributed between the two
sides. Aless ordered (more dispersed)
arrangement is more probable than a highly ordered arrangement. Entropy is
definedin terms of the number of possible arrangements of molecules.
Boltzmann’s
equation for entropy, S, is S = k
ln W. In this equation, the term W represents the number of possible arrangements
of molecules, ln is the logarithm to the base e, and k is the constant
universally referred to as Boltzmann’s constant. It is equal to R/N
where R is the gas constant and N is Avogadro’s number (6.02 × 1023), the
number of molecules in a mole.
Related Topics