Chapter: Biochemistry: Biochemistry and the Organization of Cells
How were biomolecules likely to have formed on the early Earth?
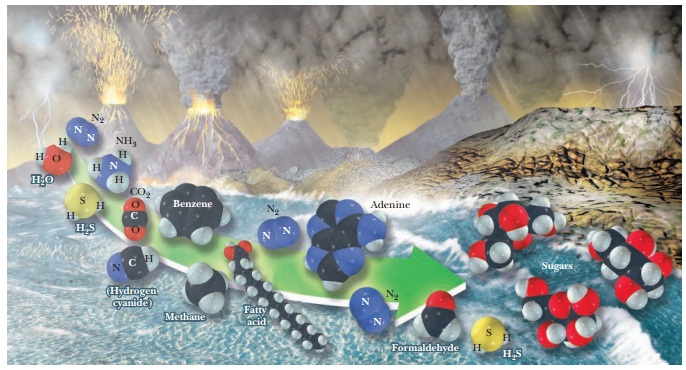
Biomolecules
How were biomolecules likely to
have formed on the early Earth?
Experiments
have been performed in which the simple compounds of the early atmosphere were
allowed to react under the varied sets of conditions that might have been
present on the early Earth. The results of such experiments indicate that these
simple compounds react abiotically
or, as the word indicates (a, “not,”
and bios, “life”), in the absence of
life, to give rise to biologically important compounds such as the components
of proteins and nucleic acids. Of historic interest is the well-known
Miller–Urey experiment, shown schemati-cally in Figure 1.4. In each trial, an electric
discharge, simulating lightning, is
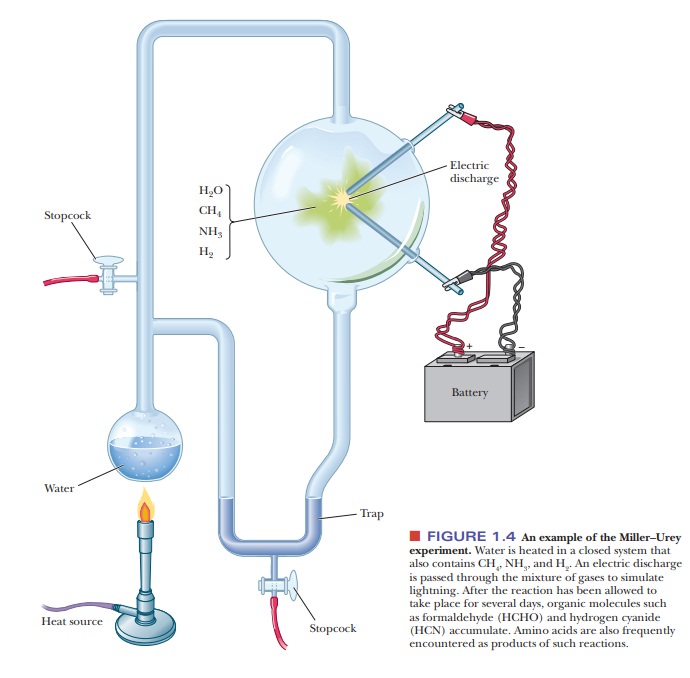
Simple
organic molecules, such as formaldehyde (HCHO) and hydrogen cyanide (HCN), are
typical products of such reactions, as are amino acids, the building blocks of
proteins. According to one theory, reactions such as these took place in the
Earth’s early oceans; other researchers postulate that such reactions occurred
on the surfaces of clay particles that were present on the
It is certainly true that mineral
substances similar to clay can serve as catalysts in many types of reactions.
Both theories have their proponents, and more research is needed to answer the
many questions that remain.
Living cells today are assemblages that include
very large molecules, such as proteins, nucleic acids, and polysaccharides.
These molecules are larger by many powers of ten than the smaller molecules
from which they are built. Hundreds or thousands of these smaller molecules, or
monomers, can be linked to produce
macromolecules, which are also called polymers.
The versatility of carbon is important here. Carbon is tetravalent and able to
form bonds with itself and with many other elements, giving rise to different
kinds of monomers, such as amino acids, nucleotides, and monosaccharides
(sugar monomers).
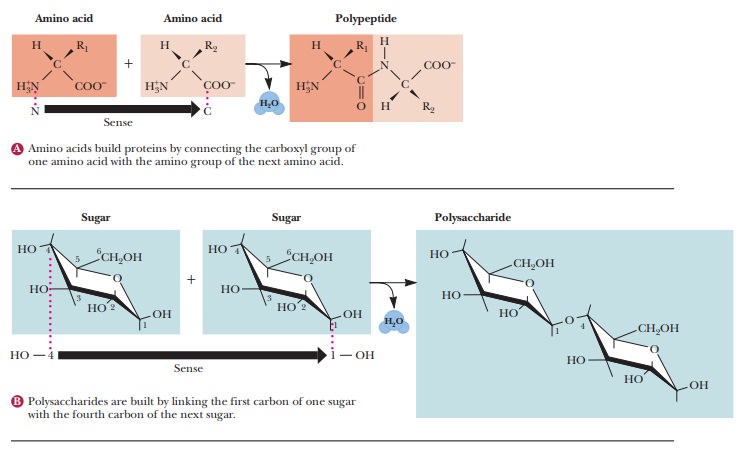
In
present-day cells, amino acids (the monomers) combine by polymeriza-tion to
form proteins, nucleotides (also
monomers) combine to form nucleicacids, and
the polymerization of sugar monomers produces polysaccharides.Polymerization
experiments with amino acids carried out under early-Earth conditions have
produced proteinlike polymers. Similar experiments have been done on the
abiotic polymerization of nucleotides and sugars, which tends to happen less
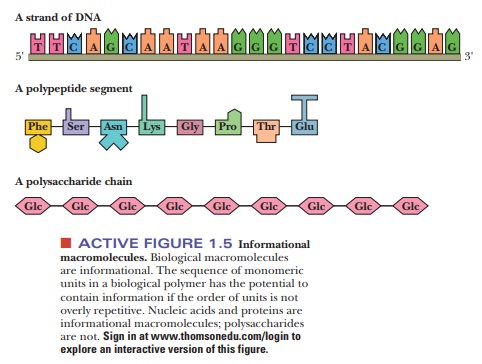
readily than thepolymerization of amino acids. Proteinsand nucleic acids play a key role in life processes.
The
several types of amino acids and nucleotides can easily be distin-guished from
one another. When amino acids form polymers, with the loss of water
accompanying this spontaneous process, the sequence of amino acids determines
the properties of the protein formed. Likewise, the genetic code lies in the
sequence of monomeric nucleotides that polymerize to form nucleic acids, the
molecules of heredity (Figure 1.5). In polysaccharides, however, the order of
monomers rarely has an important effect on the properties of the polymer, nor
does the order of the monomers carry any genetic information. (Other aspects of
the linkage between monomers are
important in polysaccha-rides, as we shall see when we discuss carbohydrates).
Notice that all the building blocks have a “head” and a “tail,” giving a sense
of direction even at the monomer level (Figure 1.6).
The
effect of monomer sequence on the properties of polymers can be illustrated by
another example. Proteins of the class called enzymes display catalytic
activity, which means that they increase the rates of chemical reac-tions
compared with uncatalyzed reactions. In the context of the origin of life,
catalytic molecules can facilitate the production of large numbers of complex
molecules, allowing for the accumulation of such molecules. When a large group
of related molecules accumulates, a complex system arises with some of the
characteristics of living organisms. Such a system has a nonrandom organization,
tends to reproduce itself, and competes with other systems for the simple
organic molecules present in the environment. One of the most important
functions of proteins is catalysis,
and the catalytic effectiveness of a given enzyme depends on its amino acid
sequence. The specific sequence of the amino acids present ultimately
determines the properties of all types of proteins, including enzymes. If not
for protein catalysis, the chemical reactions that take place in our bodies
would be so slow as to be useless for life processes.
In
present-day cells, the sequence of amino acids in proteins is determined by the
sequence of nucleotides in nucleic acids. The process by which genetic
information is translated into the amino acid sequence is very complex. DNA(deoxyribonucleic acid), one of the
nucleic acids, serves as the coding mate-rial. The genetic code is the relationship between the nucleotide sequence in
nucleic acids and the amino acid sequence in proteins. As a result of this
rela-tionship, the information for the structure and function of all living
things is passed from one generation to the next. The workings of the genetic
code are no longer completely mysterious, but they are far from completely
understood. Theories on the origins of life consider how a coding system might
have devel-oped, and new insights in this area could shine some light on the
present-day genetic code.
Related Topics