Chapter: Biochemistry: Biochemistry and the Organization of Cells
Chemical Foundations of Biochemistry
Chemical Foundations of
Biochemistry
Organic chemistry is the study of compounds of
carbon and hydrogen andtheir derivatives. Because the cellular apparatus of
living organisms is made up of carbon compounds, biomolecules are part of the
subject matter of organic chemistry. Additionally, many carbon compounds are
not found in any organ-ism, and many topics of importance to organic chemistry
have little connection with living things. We are going to concentrate on the
aspects of organic chem-istry that we need to understand what goes on in living
cells.
Can a chemist make the molecules of life in a laboratory?
Until
the early part of the 19th century, there was a widely held belief in “vital
forces,” forces presumably unique to living things. This belief included the
idea that the compounds found in living organisms could not be produced in the
laboratory. German chemist Friedrich Wöhler performed the critical experi-ment
that disproved this belief in 1828. Wöhler synthesized urea, a well-known waste
product of animal metabolism, from ammonium cyanate, a compound obtained from
mineral (i.e., nonliving) sources.

It has
subsequently been shown that any compound that occurs in a living organism can
be synthesized in the laboratory, although in many cases the synthesis
represents a considerable challenge to even the most skilled organic chemist.
The
reactions of biomolecules can be described by the methods of organic chemistry,
which requires the classification of compounds according to their functional groups. The reactions of molecules are based on the reactions of their
respec-tive functional groups.
What makes biomolecules special?
Table 1.1 lists some biologically important functional groups. Note that most of these functional groups contain oxygen and nitrogen, which are among the most electronegative elements. As a result, many of these functional groups are polar, and their polar nature plays a crucial role in their reactivity. Some groups that are vitally important to organic chemists are missing from the table because molecules containing these groups, such as alkyl halides and acyl chlorides, do not have any particular applicability in biochemistry.
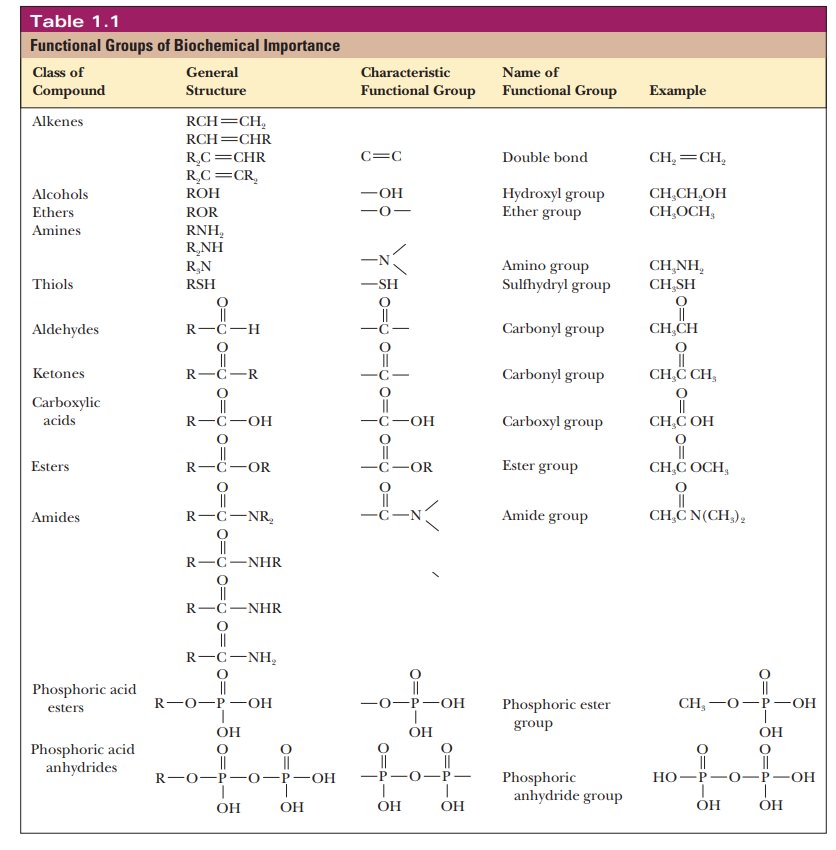
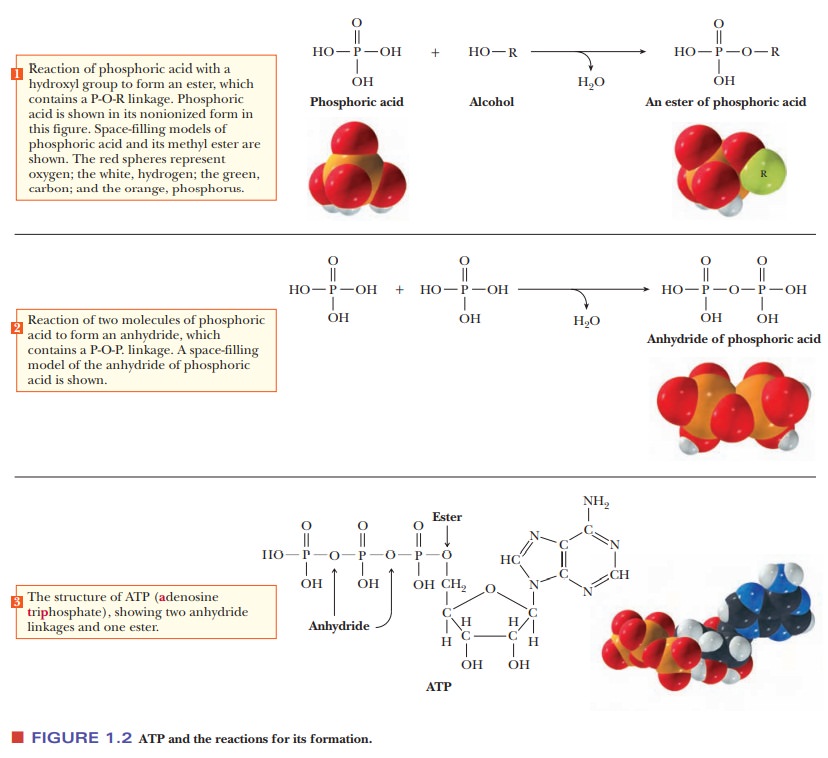
Conversely, carbon-containing
derivatives of phosphoric acid are mentioned infrequently in beginning courses
on organic chemistry, but esters and anhydrides of phos-phoric acid (Figure
1.2) are of vital importance in biochemistry. Adenosine triphosphate (ATP), a
molecule that is the energy currency of the cell, contains both ester and
anhydride linkages involving phosphoric acid.
Important
classes of biomolecules have characteristic functional groups that determine
their reactions. We shall discuss the reactions of the functional groups when
we consider the compounds in which they occur.
Summary
Life is based on compounds of carbon. This is
the subject matter of organic chemistry.
The
reactions of organic compounds are those of their functional groups, which are
specifically linked atoms that react in similar ways under many different
conditions.
Related Topics