Chapter: Biochemistry: Biochemistry and the Organization of Cells
Biochemical Energetics
Biochemical Energetics
What is the source of energy in life processes?
All
cells require energy for a number of purposes. Many reactions that take place
in the cell, particularly those involving synthesis of large molecules, can-not
take place unless energy is supplied. The Sun is the ultimate source of energy
for all life on Earth. Photosynthetic organisms trap light energy and use it to
drive the energy-requiring reactions that convert carbon dioxide and water to
carbohydrates and oxygen. (Note that these reactions involve the chemical
process of reduction.)
Nonphotosynthetic organisms, such as animals that consume these carbohydrates,
use them as energy sources. (The reactions that release energy involve the
chemical process of oxidation.) We
are going to dis-cuss the roles that oxidation and reduction reactions play in
cellular processes, and you will see many examples of such reactions in
subsequent. For the moment, it is useful and sufficient to recall from general
chemistry that oxidation is the loss of
electrons and reduction is the gain of electrons.
How do we measure energy changes in biochemistry?
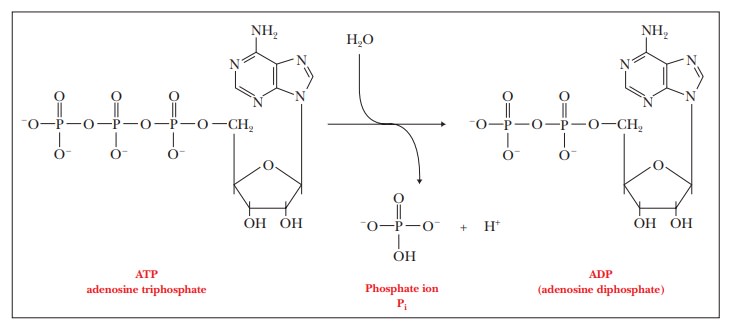
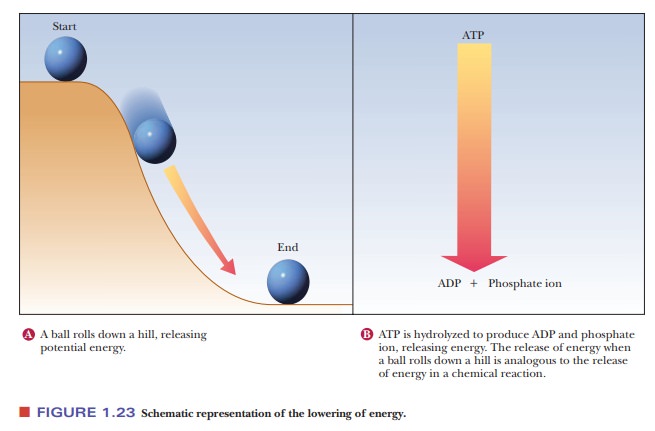
One of the most important questions about any process is whether energy changes favor the process. Thermodynamics is the branch of science that deals with this question. The key point is that processes that release energy are favored. Conversely, processes that require energy are disfavored. The change in energy depends only on the state of the molecules present at the start of the process and the state of those present at the end of the process. This is true whether the process in question is the formation or breaking of a bond, the formation or disruption of an intermolecular interaction, or any possible process that requires or can release energy. This material is of central importance, and it tends to be challenging for many. What we say about it now will make it easier to apply in later. A reaction that takes place as a part of many biochemical processes is the hydrolysis of the compound adenosine triphosphate, or ATP.
Summary
The Sun is the source of energy for all life on Earth. It provides
the energy for photosynthesis, which produces carbohydrates as well as
oxy-gen. Carbohydrates can be processed in chemical reactions that release
energy.
Reactions that release energy are favored and thus are likely to
occur. Thermodynamics is the branch of science that predicts the
likelihood of reactions.
Related Topics