Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Physical and chemical equilibrium
Physical
and chemical equilibrium
Introduction
In
our daily life, we observe several chemical and physical changes. For example,
a banana gets ripened after few days, silver gets tarnished in few months and
iron gets rusted slowly. These processes proceed in one direction. Now let us
consider the transport of oxygen by hemoglobin in our body as an illustration
for a reversible change. The hemoglobin combines with oxygen in lungs to form
oxyhemoglobin. The oxy-hemoglobin has a tendency to form hemoglobin by
releasing oxygen. In fact, in our lungs all the three species coexist.
Few
chemical reactions proceed in only one direction whereas many reactions proceed
in both the directions and these reactions are called reversible reactions.
In
chemical reactions, the concentration of the reactants decreases and that of
the products increases with time. In reversible reactions, initially the
reaction proceeds towards the formation of the product. Upon formation of the
product, the reverse reaction begins to take place. At a particular stage, the
rate of the reverse reaction is equal to that of the forward reaction
indicating a state of equilibrium.
It
is desirable to know the three crucial aspects of chemical reactions namely the
feasibility, the rate of the reaction and the extent of reaction. We know that
the feasibility of a reaction is given by thermodynamics. Chemical kinetics
will tell about the rate of the reaction. The equilibrium constant tells about
the extent of a reaction which we will discuss in this chapter. We will also
discuss the types of equilibrium, the significance of equilibrium constant and
its relationship to thermodynamic quantities and the response of chemical
equilibrium to change in the reaction conditions.
Physical
and chemical equilibrium:
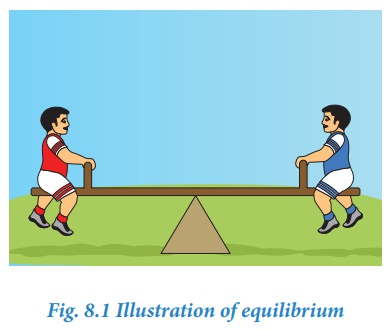
There
are different types of equilibrium. For example, if two persons with same
weight sit on opposite sides of a see-saw at equal distance from the fulcrum,
then the see-saw will be stationary and straight and it is said to be in
equilibrium.
Another
example of a state of equilibrium is the game of "tug-of-war." In
this game a rope is pulled taut between two teams. There may be a situation
when both the teams are pulling the rope with equal force and the rope is not
moving in either direction. This state is said to be in equilibrium.

In
reversible processes, the rate of two opposing reactions equals at a particular
stage. At this stage the concentration of reactants and products do not change
with time. This condition is not static and is dynamic, because both the
forward and reverse reactions are still occurring with the same rate.
Related Topics