Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Brief questions and answers: Physical and Chemical Equilibrium
Physical and Chemical Equilibrium | Chemistry
Answer the following questions
26. If there is no change in concentration, why is the equilibrium state considered dynamic ?
●
The equilibrium constant does not indicate whether a reaction is in static or
dynamic equilibrium.
●
It is simply the concentrations of products divided by the concentrations of
reactants.
●
A reaction is dynamic equilibrium if the rate of the forward reaction is equal
to the rate of backward reaction.
27. For a given reaction at a particular temperature, the equilibrium constant has constant value. Is the value of Q also constant ? Explain.
Q
is a reaction quotient, which helps to determine if a reaction will shift
forward or backward direction. As a system approaches towards equilibrium, Q
approaches towards K.
28. What the relation between KP and KC. Give one example for which KP is equal to KC.
The
relation between equilibrium constants KP and Kc are
given by
KP= Kc (RT)Δng
Δng = 2 – 2 = 0
H2(g)
+ I2(g) → 2HI(g)
KP
= KC
29. For a gaseous homogeneous reaction at equilibrium, number of moles of products are greater than the number of moles of reactants. Is KC is larger or smaller than KP.
For
a gaseous homogenious equilibrium reaction at equilibrium, number of moles of
products are greater than the number of moles of reactants.
For
example PCl5(g) ⇌ PCl3(g)
+ Cl2(g)
Δng
= 2 − 1 = 1 positive
The
relation between KP & KC for above reaction is KP
> KC. Hence KC is smaller than KP.
30. When the numerical value of the reaction quotient (Q) is greater than the equilibrium constant (K), in which direction does the reaction proceed to reach equilibrium ?
When
the numerical value of reaction quotient Q is greater then the equilibrium
constant KC. Q > KC. The equilibrium shift to the left
and produce more reactants.
31. For the reaction,
A2(g) + B2(g) ⇌ 2AB(g) ; ΔH is –ve.
the following molecular scenes represent different reaction mixture (A – green, B – blue)
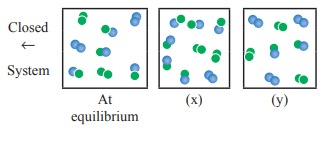
i) Calculate the equilibrium constant KP and (KC).
ii) For the reaction mixture represented by scene (x), (y) the reaction proceed in which directions ?
iii) What is the effect of increase in pressure for the mixture at equilibrium.
Solution:
i)
KC= [AB]2 / [A2] [B2]
A
– Green
B – blue
Given
that 'V' is constant (closed system)
At
equilibrium
∴KC =
[ (4/V)2 ] / [ (2/V) (2/V) ] = 16 / 4 = 4
KP
= KC (RT)Δng
KP
= 4 (RT)o = 4
ii)
At Stage 'x'
Q
= ( [AB]2 ) / ( [A2] [B2] ) = (6/V)2
/ ( (2/V) (1/V) ) = 36/2 = 18
Q
> KC ie., reverse reaction is favoured
iii)
At stage 'y'
Q
= (3/V)2 / ( (3/V)(3/V) ) = 9 / (3× 3) = 1
KC > Q ie., forward reaction is favoured.
32. State Le-Chatelier principle.
If
a system at equilibrium is disturbed, then the equilibrium shifts itselft in a
direction that nullifies the effect of that disturbance.
33. Consider the following reactions,
a) H2(g) + I2(g) ⇌ 2 HI
b) CaCO3 (s) ⇌ CaO (s) + CO2(g)
c) S(s) + 3F2 (g) ⇌ SF6 (g)
In each of the above reaction find out whether you have to increase (or) decrease the volume to increase the yield of the product.
Ans:
Δng = 2−2 = 0
a)
The number of moles of products is equal to the number of moles of reactants,
so change in pressure has no effect on equilibrium.
b)
Δng = 1− 0 = 1
Δng = +ve
(ie)
To increase the yield of product pressure should be decrease and hence volume
increases
c)
Δng = 1−3 = −2
Δng
= -ve
(ie)
pncrease in pressure favours formation of product hence volume should be
decreased
34. State law of mass action.
At
any instant, the rate of chemical reaction at a given temperature is directly
proportional to the product of the active mass of the reactants at that
instant.
35. Explain how will you predict the direction of a equilibrium reaction.
The
direction of equilibrium reaction can be predicted by comparing Q with KC.
●
Q = KC ; the reaction is in equilibrium state.
●
Q > KC ; the reaction will proceed in the reverse direction i.e
formation of reactants.
●
Q < KC, the reaction will proceed in the forward direction ie the
formation of products.
36. Derive a general expression for the equilibrium constant KP and KC for the reaction
3H2(g) + N2(g) ⇌ 2NH3(g)
Synthesis of ammonia:
Let us consider the formation of ammonia in which
'a' moles nitrogen and 'b' moles hydrogen gas are allowed to react in a
container of volume V. Let 'x' moles of nitrogen react with 3x moles of
hydrogen to give 2x moles off ammonia.
N2(g) + 3H2(g) ⇌ 2NH3(g)
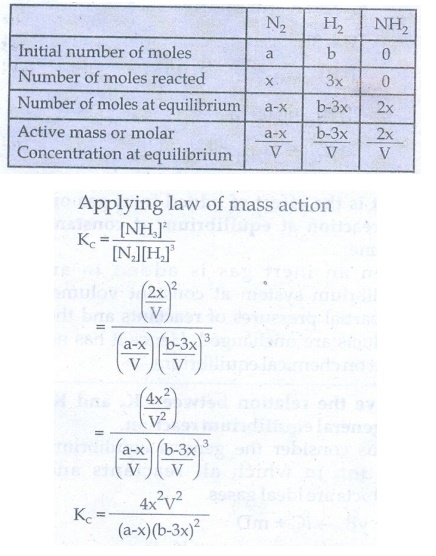
The equilibrium constant Kp can be also be calculated as follows:
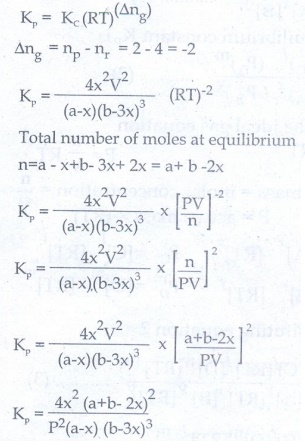
37. Write a balanced chemical equation for a equilibrium reaction for which the equilibrium constant is given by expression
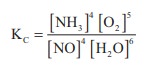
Solution:
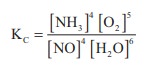
KC
= [NH3]4[O2]5 / [NO]4[H2O]6
Chemical
equation is
4NO + 6H2O ⇌ 4NH3 +5O2
38. What is the effect of added inert gas on the reaction at equilibrium
When
an inert gas is added to an equilibrium system at constant volume, the partial
pressures of reactants and the products are unchanged. Hence it has no effect
on chemical equilibrium.
39. Derive the relation between KP and KC
Let us
consider the general equilibrium reaction in which all reactants and products
are ideal gases.
xA + y B → lC + mD
The
equilibrium constant KC is
KC
= [C]l [D]m / [A]x[B]y …………….(1)
The
equilibrium constant KP is
KP
= [ (PC)l.(PD)m ] / [ (PA)x.(PB)y ]
…………….(2)
From
the ideal gas equation
PV =
nRT
P = n/V
RT
Active
mass = molar concentration = n / V
P =
active mass × (RT)
PAx
= [A]x . [RT] x
PCl
= [C] l . [RT] l
PBy
= [B]y . [RT]y
PDm
= [D]m . [RT]m
on
subsituting equation 2
kP
= { [C]l.[RT]l [D]m.[RT]m
} / { [A]x.[RT] x [B]y.[RT]y
} ………. (3)
kP
= { [C]l[D]m[RT]l+m }
/ { [A]x [B]y[RT]x+y }
kP
= { [C]l[D]m / [A]x[B]y }
[RT]( l + m) - ( x + y) …….(4)
By
comparing equation (1) & (4) we get
kP
= KC (RT) (Δng) ΔS ……….(5)
Where Δng,
is the difference between the sum of number of moles of gaseous products and
the sum of the number of moles of gaseous reactants.
40. One mole of PCl5 is heated in one litre closed container. If 0.6 mole of chlorine is found at equilibrium, calculate the value of equilibrium constant.
Solution:
Given
that [PCl5]initital = 1 mole / 1 dm3
[Cl2]eq = 0.6 mole
dm−3
PCl5 → PCl3 + Cl2
[PCl3]eq = 0.6 mole
dm−3
[PCl5]eq = 0.4 mole
dm−3
KC
= ( [PC13][C12] ) / [PCl5]
=
(0.6 × 0.6) / 0.4 = 0.9
KC
= 0.9
41. For the reaction
SrCO3 (s) ⇌ SrO (s) + CO2(g),
the value of equilibrium constant KP = 2.2 × 10–4 at 1002 K. Calculate KC for the reaction.
Solution:
For
the reaction,
SrCO3(s)
⇌ SrO (s) + CO2(g)
Δng
= 1 −
0 = 1
KP
= KC (RT)
2.2
× 10-4 = KC (0.0821) (1002)
KC
= ( 2.2× 10-4 ) / ( 0.0821× 1002 )
= 0.0267 × 10-4
= 2.67 × 10-6
42. To study the decomposition of hydrogen iodide, a student fills an evacuated 3 litre flask with 0.3 mol of HI gas and allows the reaction to proceed at 500 0C. At equilibrium he found the concentration of HI which is equal to 0.05 M. Calculate KC and KP.
Solution:
V
= 3L
[HI]initital
= 0.3 / 3 = 0.1M
[HI]eq
= 0.05 M
2HI(g)
⇌ H2(g) + I2(g)
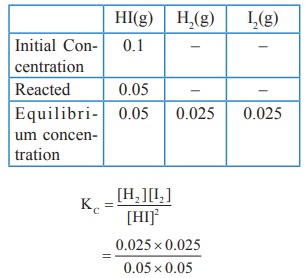
KC
= [H2] [I2] / [HI]2
=
(0.025 ×0.025) / (0.05)2
KP
= KC (RT)Δng
Δng
= 2−2 = 0
KP
= 0.25 (RT)°
∴ KP = 0.25
43. Oxidation of nitrogen monoxide was studied at 200 0C with initial pressures of 1 atm NO and 1 atm of O2. At equilibrium partial pressure of oxygen is found to be 0.52 atm calculate KP value.
Solution:
2NO(g)
+ O2(g) ⇌ 2NO2(g)
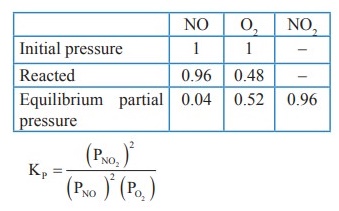
KP
= [PNO2]2/ ( [PNO]2[PO2])
= (0.96 × 0.96) / (0.04 × 0.04 × 0.52)
= 1.107 × 103 (atm)−1
44. 1 mol of CH4, 1 mole of CS2 and 2 mol of H2S are 2 mol of H2 are mixed in a 500 ml flask. The equilibrium constant for the reaction KC = 4 × 10–2 mol2 lit–2. In which direction will the reaction proceed to reach equilibrium ?
Solution:
CH4(g)
+ 2H2S(g) ⇌ CS2(g)
+ 4H2(g)
KC
= 4 ×10−2 mol2lit−2
V
= 500 ml = 1/2 L
[CH4]in
= 1 mol / (½)L = 2 mol L−1 ;
[CS2]in
= 1 mol / (½)L = 2 mol L−1
[H2S]in
= 2 mol / (½)L = 4 mol L−1
[H2]in
= 2 mol / (½)L = 4 mol L−1
Q
= ( [CS2] [H2]4 ) / ( [CH4]
[H2S]2 ) = [ 2 × (4)4 ] / [ 2 × (4)2
] = 16
Q
> KC
∴ The
reaction will proceed in the reverse direction to reach the equilibrium.
45. At particular temperature KC = 4 × 10–2 for the reaction
H2S(g) ⇌ H2(g) + ½ S2(g)
Calculate KC for each of the following reaction
i) 2H2S (g) ⇌ 2H2 (g) + S2 (g)
ii) 3H2S (g) ⇌ 3H2 (g) + 3/2 S2(g)
Solution:
i)
2H2S(g) ⇌ 2H2(g)
+ S2(g)
ii)
3H2S(g) ⇌ 3H2(g)
+ 3/2 S2(g)
KC
= 4 × 10−2 for the reaction,
H2S(g)
⇌ H2(g) + ½ S2(g).
KC
= ( [H2] [S2]½ ) / [H2S]
⇒ 4 × 10−2 = [H2][S2]
½ / [H2S]
For
the reaction,
2H2S(g)
⇌ 2H2(g)
+ S2(g)
KC
= ([H2]2[S2]) / [H2S]2
= (4 × 10−2)2
=
16 × 10-4
For
the reaction,
3H2S(g)
⇌ 3H2(g)
+ 3/2 S2(g)
KC
= ([H2]3[S2] 3/2) /
[H2S]3 = (4 × 10−2)3
= 64 × 10-6
46. 28 g of Nitrogen and 6 g of hydrogen were mixed in a 1 litre closed container. At equilibrium 17g NH3 was produced. Calculate the weight of nitrogen, hydrogen at equilibrium.
Solution:
Given,
mN2
= 28g; mH2 = 6g;
mNH3
= 17g ; V = 1 L
(nN2)initiaI
= 28 / 28 = 1 mol
(nH2)initial
= 6 / 2 = 3 mol
N2(g)
+ 3H2(g) ⇌ 2NH3(g)
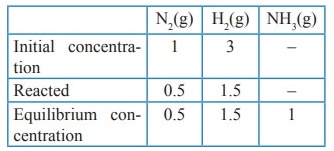
[NH3]
= (17 / 17 ) = l mol
Weight
of N2 = (no.of moles of N2) × molar mass of N2
=
0.5 × 28 =14g
Weight
of H2 = (no.of moles of H2) × molar mass of H2
=
1.5 × 2 = 3g
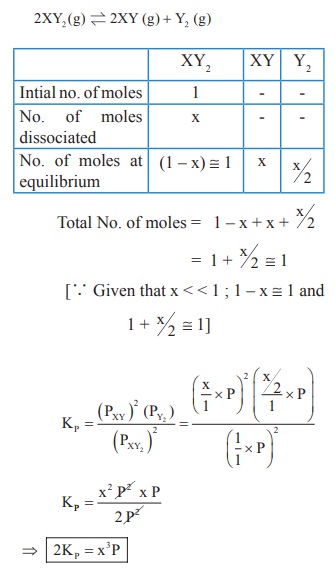
47. The equilibrium for the dissociation of XY2 is given as,
2XY2 (g) ⇌ 2XY (g) + Y2(g)
if the degree of dissociation x is so small compared to one. Show that 2 KP = PX3 where P is the total pressure and KP is the dissociation equilibrium constant of XY2.
Solution:
48. A sealed container was filled with 1 mol of A2 (g), 1 mol B2 (g) at 800 K and total pressure 1.00 bar. Calculate the amounts of the components in the mixture at equilibrium given that K = 1 for the reaction
A2 (g) + B2 (g) ⇌ 2AB (g)
Solution:
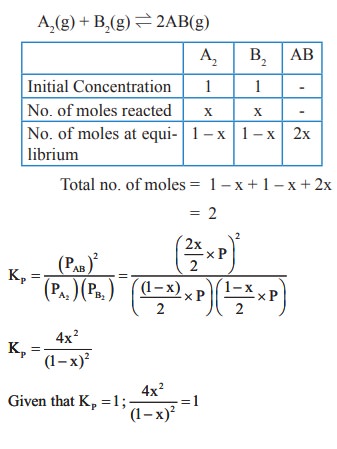
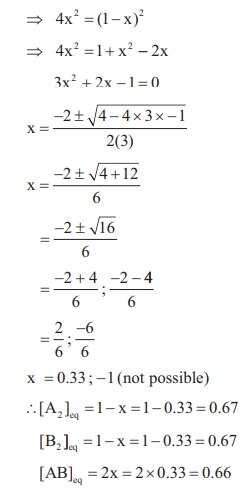
49. Deduce the Vant Hoff equation.
50. The equilibrium constant KP for the reaction
N2(g) + 3H2(g) ⇌ 2NH3(g) is 8.19 × 102 at 298 K and 4.6 × 10–1 at 498 K. Calculate ΔH0 for the reaction.
Solution:
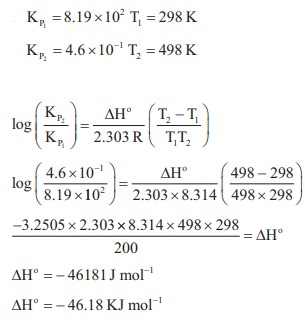
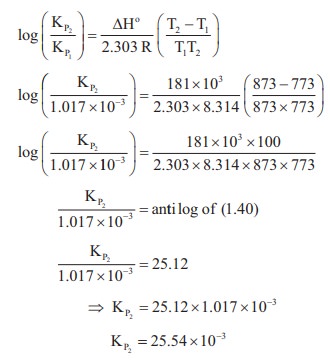
51. The partial pressure of carbon dioxide in the reaction CaCO3 (s) ⇌ CaO (s) + CO2(g) is 1.017 × 10–3 atm at 5000 C. Calculate KP at 6000c C for the reaction. ΔH for the reaction is 181 KJ mol–1 and does not change in the given range of temperature.
Solution:
PCO2 = 1.017x10-3 atm T = 500º C
Kp = PCO2
KP1 = 1.017x10-3 T=500+273=773K
KP2 = ? T=600+273=873K
∆Hº = 181KJmol-1
Related Topics