Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Law of mass action
Law
of mass action
In
1864 two Norwegian chemists namely Maximilian Guldberg and Peter Waage
formulated the law of mass action, based on the experimental studies of many
reversible reactions. The law states that,
“At
any instant, the rate of a chemical reaction at a given temperature is directly
proportional to the product of the active masses of the reactants at that
instant”.
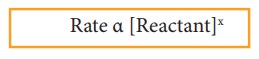
Rate α [Reactant]x
where,
x is the stoichiometric coefficient of the reactant and the square bracket
represents the active mass (concentration) of the reactants.
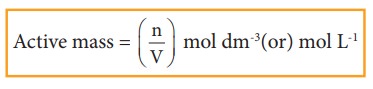
where
n is the number of moles and V is the volume of the container (dm3
or L)
Equilibrium constants (Kp and KC):
Let
us consider a reversible reaction,
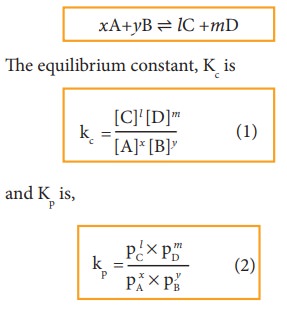
xA+yB ⇌ lC + mD
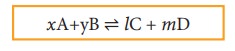
where,
A and B are the reactants, C and D are the products and x,y, l and m are the
stoichiometric coefficients of A, B, C and D, respectively.
Applying the law of mass action, the rate of the forward reaction,
rf α [A]x [B]y (or) rf
= kf [A]x [B]y
Similarly,
the rate of the backward reaction,
rb α [C]l [D]m
(or)
rb = kb [C]l[D]m
where
kf and kb are proportionality constants
At
equilibrium,
Rate of forward reaction (rf) = Rate of backward
reaction (rb)
kf [A]x [B]y
= kb [C]l [D]m
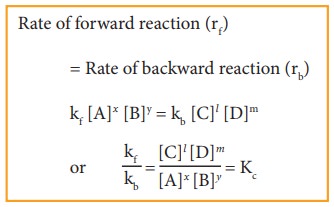
where,
Kc is the equilibrium constant in terms of concentration (active
mass).
At
a given temperature, the ratio of the product of active masses of reaction
products raised to the respective stoichiometric coefficients in the balanced
chemical equation to that of the reactants is a constant, known as equilibrium
constant. Later when we study chemical kinetics we will learn that this is only
approximately true.
If
the reactants and products of the above reaction are in gas phase, then the
equilibrium constant can be written in terms of partial pressures as indicated
below,
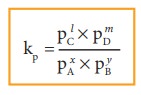
Where,
pA, pB, pC,and pD are the partial
pressures of the gas A, B, C and D, respectively.
Relation
between Kp and Kc
Let
us consider the general reaction in which all reactants and products are ideal
gases.
The
ideal gas equation is
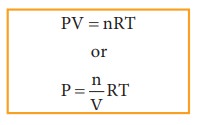
Since
Active mass = molar concentration = n/V
P = active mass × RT
Based
on the above expression the partial pressure of the reactants and products can
be expressed as,
pAx = [A]x
[RT]x
pBy = [B]y
[RT]y
pC1 = [C]l
[RT]l
pmD
= [D]m
[RT]m
On
substitution in Eqn. 2,

By
comparing equation (1) and (4), we get
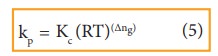
where,
Δng
is the difference between the sum of number of moles of products and the sum of
number of moles of reactants in the gas phase.
The
following relations become immediately obvious.
When
Δng = 0
Kp
= Kc (RT)0 = Kc
Example:
H2(g)
+ I2(g) ⇌ 2HI (g)
N2
(g) +O2 (g) ⇌ 2NO(g)
When
Δng = +ve
Kp
= Kc (RT)+ve
Kp>Kc
2NH3(g)
⇌ N2 (g) + 3H2 (g)
PCI5
(g) ⇌ PCl3 (g) + Cl2 (g)
When
Δng = -ve
Kp
= Kc (RT)-ve
Kp<
Kc
Example:
2H2(g)
+ O2(g) ⇌ 2H2O (g)
2SO2(g)
+ O2(g) ⇌ 2SO3 (g)
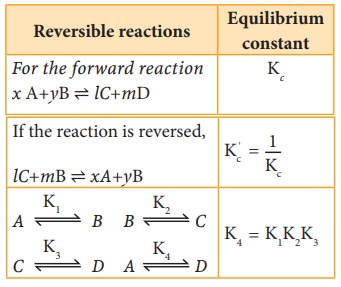
Table 8.1 Relation between equilibrium constants for some reversible reactions
Equilibrium constants for heterogeneous equilibrium
Consider
the following heterogeneous equilibrium.
CaCO3 (s) ⇌
CaO (s) + CO2 (g)
The
equilibrium constant for the above reaction can be written as
Kc = [CaO(s)][CO2(g)] / CaCO3 (s)
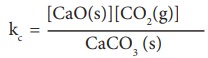
A
pure solid always has the same concentration at a given temperature, as it does
not expand to fill its container. i.e. it has same number of moles L-1
of its volume. Therefore, the concentration of a pure solid is a constant. The
above expression can be modified as follows
Kc = [CO2
(g)]
Or
Kp = pCO2
The
equilibrium constant for the above reaction depends only the concentration of
carbon dioxide and not the calcium carbonate or calcium oxide. Similarly, the
active mass (concentration) of the pure liquid does not change at a given
temperature. Consequently, the concentration terms of pure liquids can also be
excluded from the expression of the equilibrium constant.
For
example,
CO2(g) + H2O (l) ⇌ H+(aq) + HCO3–
(aq)
Since,
H2O (l) is a pure liquid the Kc can be expressed as
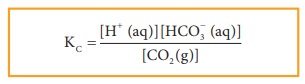
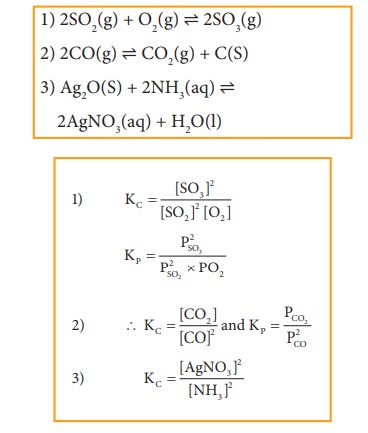
Example
Write
the Kp and Kc for the following reactions
Related Topics