Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Physical equilibrium
Physical and chemical equilibrium:
There
are different types of equilibrium. For example, if two persons with same
weight sit on opposite sides of a see-saw at equal distance from the fulcrum,
then the see-saw will be stationary and straight and it is said to be in
equilibrium.
Another
example of a state of equilibrium is the game of "tug-of-war." In
this game a rope is pulled taut between two teams. There may be a situation
when both the teams are pulling the rope with equal force and the rope is not
moving in either direction. This state is said to be in equilibrium.

In
reversible processes, the rate of two opposing reactions equals at a particular
stage. At this stage the concentration of reactants and products do not change
with time. This condition is not static and is dynamic, because both the
forward and reverse reactions are still occurring with the same rate.
Physical
equilibrium
A
system in which the amount of matter constituting different phases does not
change with time is said to be in physical equilibrium. This involves no
perceptible physical change in the system. To understand the physical
equilibrium let us analyse the following phase changes.
Solid-liquid equilibrium
Let
us consider the melting of ice in a closed container at 273 K. This system will
reach a state of physical equilibrium in which the amount of water in the solid
phase and liquid phase does not change with time. In the process the total
number of water molecules leaving from and returning to the solid phase at any
instant are equal.
If
some ice-cubes and water are placed in a thermos flask (at 273K and 1 atm
pressure), then there will be no change in the mass of ice and water.
At
equilibrium,
Rate
of melting of ice = Rate of freezing of water
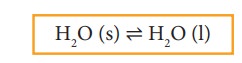
The
above equilibrium exists only at a particular temperature and pressure. The
temperature at which the solid and liquid phases of a substance are at
equilibrium is called the melting point or freezing point of that substance.
Liquid - Vapour equilibrium
Similarly,
there exists an equilibrium between the liquid phase and the vapour phase of a
substance. For example, liquid water is in equilibrium with its vapour at 373 K
and1 atm pressure in a closed vessel.
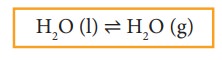
Here
Rate
of evaporation = Rate of condensation
The
temperature at which the liquid and vapour phases are at equilibrium is called
the boiling point and condensation point of the liquid.
Solid - Vapour equilibrium
Consider
a system in which the solid sublimes to vapour. In this process also,
equilibrium can be established between these two phases. When solid iodine is
placed in a closed transparent vessel, after sometime,the vessel gets filled up
with violet vapour due to sublimation of iodine. Initially, the intensity of
the violet colour increases, after sometime it decreases and finally it becomes
constant, as the following equilibrium is attained.
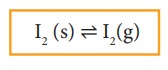
More examples
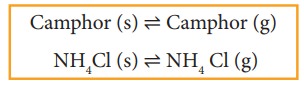
Equilibrium involving dissolution of solids or gases in liquids
Solid in liquids
When
you add sugar to water at a particular temperature, it dissolves to form sugar
solution. If you continue to add much sugar, you will reach a stage at which
the added sugar remains as solid and the resulting solution is called a
saturated solution. Here, as in the previous cases a dynamic equilibrium is
established between the solute molecules in the solid phase and in the solution
phase.
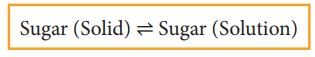
In
this process
Rate of dissolution of solute = Rate of crystallisation of
solute
Gas in liquids
When
a gas dissolves in a liquid under a given pressure, there will be an
equilibrium between gas molecules in the gaseous state and those dissolved in
the liquid.
Example:
In
carbonated beverages the following equilibrium exists.
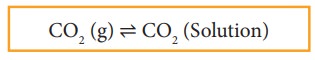
Henry’s
law is used to explain such gas-solution equilibrium processes.
Related Topics