Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Homogeneous and heterogeneous equilibria
In a homogeneous equilibrium, all the reactants and products are in the same phase.
Homogeneous
and heterogeneous equilibria
Homogeneous equilibrium
In
a homogeneous equilibrium, all the reactants and products are in the same
phase.
For example:
H2 (g) + I2 (g) ⇌ 2HI (g)
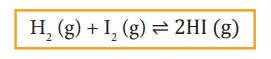
In
the above equilibrium, H2, I2 and HI are in the gaseous
state.
Similarly,
for the following reaction, all the reactants and products are in homogeneous
solution phase.
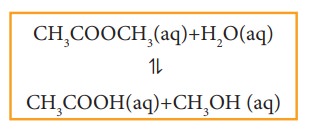
Heterogeneous equilibrium
If
the reactants and products of a reaction in equilibrium, are in different
phases, then it is called as heterogeneous equilibrium.
Example:
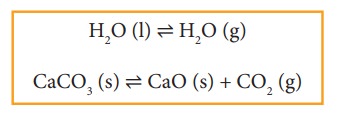
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium : Homogeneous and heterogeneous equilibria |
Related Topics
11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium