Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Dynamic nature of equilibrium
Dynamic
nature of equilibrium:
Let
us consider, a situation in multi storey building, people are moving from first
floor to second floor and vice versa. Assume that a certain number people moves
up from first floor to second floor in a specific time, and the same number of
people moves down from second floor to the first floor in the same time. Now,
the rate of movement of people from first to second floor equals the rate of
movement of people from second to first floor, and hence the number of people
in each floor will remain the same. Thus the population of people on the two
floors is in a dynamic equilibrium Let us extend this analogy to understand
dynamic nature of equilibrium.
Chemical reactions which are reversible do not cease, when equilibrium is attained. At equilibrium the forward and the backward reactions are proceeding at the same rate and no macroscopic change is observed. So chemical equilibrium is in a state of dynamic equilibrium.
For example,
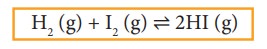
Related Topics