Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Application of equilibrium constant
Application
of equilibrium constant
The
knowledge of equilibrium constant helps us to
1.
predict the direction in which the net reaction will take place
2.
predict the extent of the reaction and
3.
calculate the equilibrium concentrations of the reactants and products.
It
is to be noted that these constants do not provide any information regarding
the rates of the forward or reverse reactions.
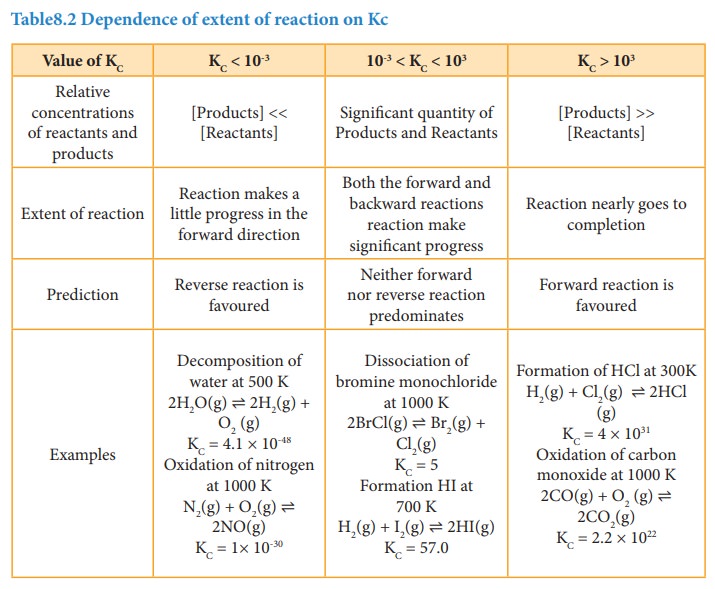
1. Predicting the extent of a reaction
The
value of equilibrium constant, Kc tells us the extent of a reaction,
i.e., it indicates how far the reaction has proceeded towards product formation
at a given temperature.
A
large value of Kc indicates that the reaction reaches equilibrium
with high product yield. On the other hand, a low value of Kc
indicates that the reaction reaches equilibrium with low product formed. In
general, if the Kc is greater than the 103, the reaction
proceeds nearly to completion. If it is less than 10-3, the reaction
rarely proceeds. If the Kc is in the range 10-3 to103,
significant amount of both reactants and products are present at equilibrium.
Table8.2 Dependence of extent of reaction on Kc
Example
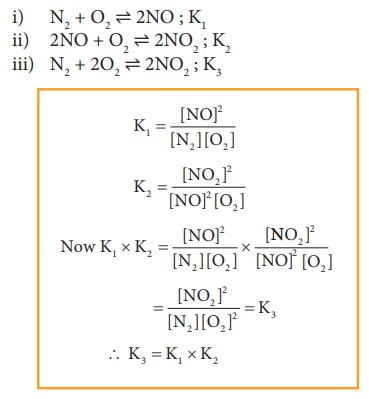
Consider
the following equilibrium reactions and relate their equilibrium, constants
2. Predicting the direction of a reaction
From
the knowledge of equilibrium constant, it is possible to predict the direction
in which the net reaction is taking place for a given concentration or partial
pressure of reactants and products.
Consider
a general homogeneous reversible reaction,
xA + yB ⇌ lC + mD
For
the above reaction under non-equilibrium conditions, reaction quotient ‘Q’ is
defined as the ratio of the product of active masses of reaction products
raised to the respective stoichiometric coefficients in the balanced chemical
equation to that of the reactants.
Under
non-equilibrium conditions, the reaction quotient Q can be calculated using the
following expression.
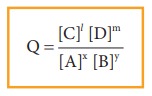
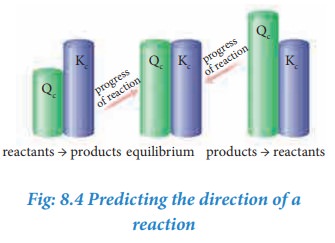
As
the reaction proceeds, there is a continuous change in the concentration of
reactants and products and also the Q value until the reaction reaches the
equilibrium. At equilibrium Q is equal to Kc at a particular
temperature. Once the equilibrium is attained, there is no change in the Q
value. By knowing the Q value, we can predict the direction of the reaction by
comparing it with Kc.
·
If
Q = Kc, the reaction is in equilibrium state.
·
If
Q > Kc, the reaction will proceed in the reverse direction i.e.,
formation of reactants.
·
If
Q < Kc, the reaction will proceed in the forward direction i.e.,
formation of products.
Example 1
The
value of Kc for the following reaction at 717 K is 48.
H2(g) + I2(g) ⇌ 2HI(g)
At
a particular instant, the concentration of H2, I2and HI
are found to be 0.2 mol L-1, 0.2 mol L-1 and 0.6 mol L-1
respectively. From the above information we can predict the direction of
reaction as follows.
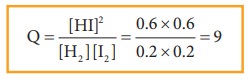
Since Q< Kc, the reaction will proceed in the forward direction.
Example 2
The
value of Kc for the reaction
N2O4 (g) ⇌
2NO2(g)
Kc
= 0.21 at 373 K. The concentrations N2O4 and NO2
are found to be 0.125 mol dm-3 and 0.5 mol dm-3 respectively at a
given time. From the above information we can predict the direction of reaction
as follows.
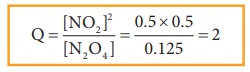
The
Q value is greater than Kc. Hence, the reaction will proceed in the reverse
direction until the Q value reaches 0.21
3. Calculation of concentration of reactants and products at equilibrium
If
the equilibrium concentrations of reactants and products are known for a
reaction, then the equilibrium constant can be calculated and vice versa.
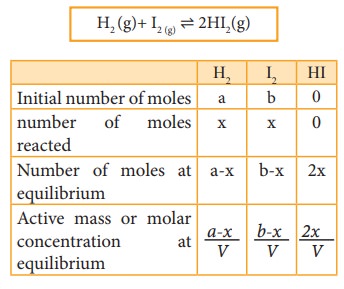
Let
us consider the formation of HI in which, ‘a’ moles of hydrogen and ‘b’ moles
of iodine gas are allowed to react in a container of volume V. Let ‘x’ moles of
each of H2 and I2 react together to form 2x moles of HI.
H2 (g)+ I2 (g) ⇌ 2HI2(g)
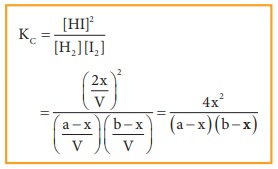
Applying
law of mass action,
The
equilibrium constant Kp can also be calculated as follows:
We
know the relationship between the Kc and Kp

Solved Problem
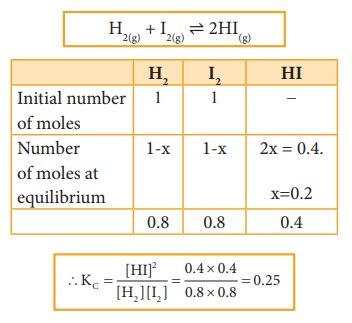
One
mole of H2 and one mole of I2 are allowed to attain
equilibrium. If the equilibrium mixture contains 0.4 mole of HI. Calculate the
equilibrium constant.
Given data:
[H2] = 1 mole [I2] = 1 mole
At
equilibrium, [HI] = 0.4 mole Kc= ?
Solution:
Dissociation of PCl5:
Consider
that ‘a’ moles of PCl5 is taken in a container of volume V. Let ‘x’
moles of PCl5 be dissociated into x moles of PCl3 and x
moles of Cl2.
PCl5(g) ⇌
PCl3(g) + Cl2(g)
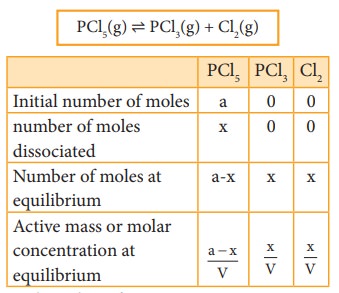
Applying
law of mass action,
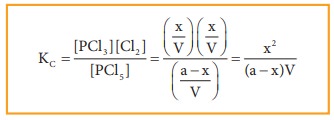
The
equilibrium constant Kp can also be calculated as follows:
We
know the relationship between the Kc and Kp
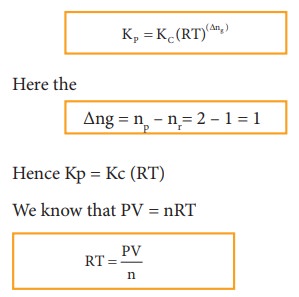
Where
n is the total number of moles at equilibrium.
n
= (a-x) + x + x = (a+x)
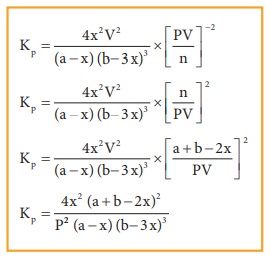
Synthesis of ammonia:
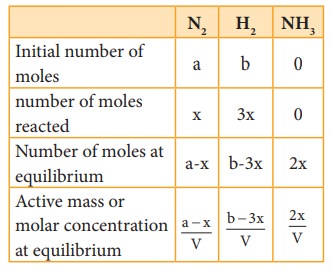
Let
us consider the formation of ammonia in which, ‘a’ moles nitrogen and ‘b’ moles
hydrogen gas are allowed to react in a container of volume V. Let ‘x’ moles of
nitrogen react with 3x moles of hydrogen to give 2x moles of ammonia.
N2(g)
+ 3H2(g) ⇌ 2NH3(g)
Applying
law of mass action,
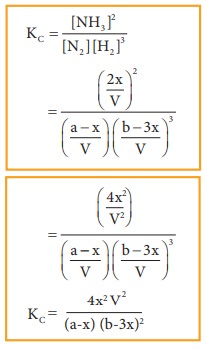
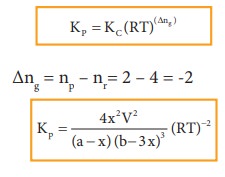
The
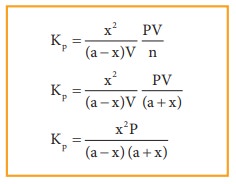
equilibrium constant Kp can also be calculated as follows:
Total
number of moles at equilibrium,
n=
a-x + b-3x+ 2x = a+b-2x
Solved Problems:
1.
The equilibrium concentrations of NH3, N2 and H2
are 1.8 × 10-2 M, 1.2 × 10-2 M and 3 × 10-2 M
respectively. Calculate the equilibrium constant for the formation of NH3
from N2 and H2. [Hint: M= mol lit-1]
Given data:
[NH3]
= 1.8 × 10-2 M
[N2]
= 1.2 × 10-2 M
[H2]
= 3 × 10-2 M
Kc
= ?
Solution:
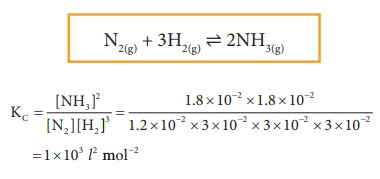
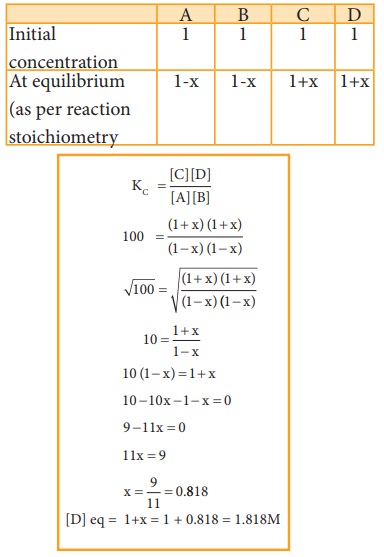
2.
The equilibrium constant at 298 K for a reaction is 100.
A
+ B ⇌ C + D
If
the initial concentration of all the four species is 1 M, the equilibrium
concentration of D (in mol lit-1) will be
Given data:
[A]
= [B] = [C]= [D] =1 M
Kc
= 100
[D]eq
= ?
Solution:
Let
x be the no moles of reactants reacted
Related Topics