Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Oxidation Number
Oxidation Number:
It is defined as the imaginary charge left on the atom when all other atoms of the compound have been removed in their usual oxidation states that are assigned according to set of rules. A term that is often used interchangeably with oxidation number is oxidation state
1) The oxidation state of a free element (i.e. in its uncombined state) is zero.
Example : each atom in H2, Cl2, Na, S8 have the oxidation number of zero.
2) For a monatomic ion, the oxidation state is equal to the net charge on the ion.
Example : The oxidation number of sodium in Na+ is +1.
The oxidation number of chlorine in Cl – is –1.
3) The algebric sum of oxidation states of all atoms in a molecule is equal to zero, while in ions, it is equal to the net charge on the ion.
Example:
In H2SO4, 2 × (oxidation number of hydrogen) + (oxidation number of S)+ 4 (oxidation number of oxygen) = 0 .
In SO42–, (oxidation number of S) + 4 (oxidation number of oxygen) = – 2.
4) Hydrogen has an oxidation number of +1 in all its compounds except in metal hydrides where it has – 1 value.
Example:
Oxidation number of hydrogen in hydrogen chloride (HCl) is + 1.
Oxidation number of hydrogen in sodium hydride (NaH) is –1.
5) Fluorine has an oxidation state of – 1 in all its compounds.
6) The oxidation state of oxygen in most compounds is –2. Exceptions are peroxides, super oxides and compounds with fluorine.
Example : Oxidation number of oxygen,
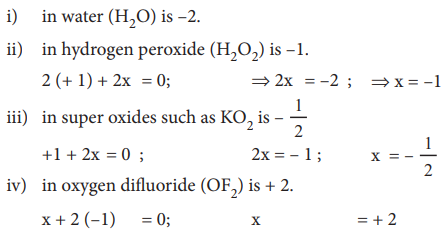
7) Alkali metals have an oxidation state of + 1 and alkaline earth metals have an oxidation state of + 2 in all their compounds.
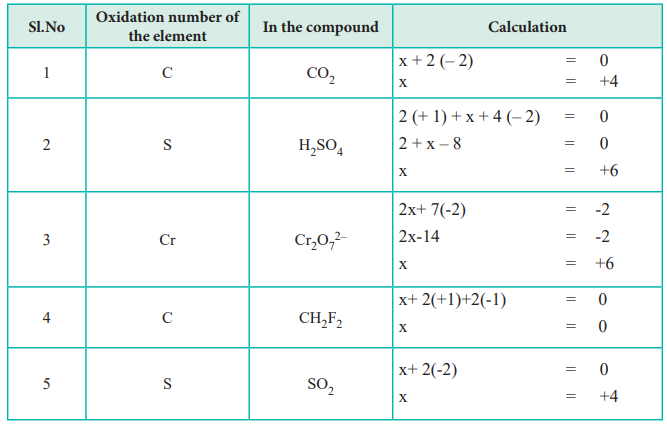
Calculation of oxidation number using the above rules.
Redox reactions in terms of oxidation numbers
During redox reactions, the oxidation number of elements changes. A reaction in which oxidation number of the element increases is called oxidation. A reaction in which it decreases is called reduction.
Consider the following reaction
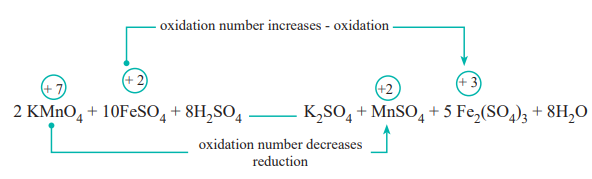
In this reaction, manganese in potassium permanganate (KMnO4) favours the oxidation of ferrous sulphate (FeSO4) into ferric sulphate (Fe2(SO4) 3 by gaining electrons and thereby gets reduced. Such reagents are called oxidising agents or oxidants. Similarly the reagents which facilitate reduction by releasing electrons and get oxidised are called reducing agents.
Related Topics