Solved Example Problems - Evaluate Yourself: Basic Concepts of Chemistry and Chemical Calculations | 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Evaluate Yourself: Basic Concepts of Chemistry and Chemical Calculations
Evaluate Yourself
1) By applying the knowledge of
chemical classification, classify each of the following into elements,
compounds or mixtures.
(i) Sugar
(ii) Sea water
(iii) Distilled water
(iv) Carbon dioxide
(v) Copper wire
(vi) Table salt
(vii) Silver plate
(viii) Naphthalene balls
Solution:
(i) Element - Copper wire,
Silver plate
(ii)
Compound - Sugar, distilled water, carbon dioxide, Table
salt, Naphthalene balls
(iii) Mixture - Sea water
Evaluate Yourself
2. Calculate the molar mass of the
following.
Ethanol(C2H5OH)
Potassium permanganate (KMnO4)
Potassium dichromate (K2Cr2O7)
Sucrose (C12H22O11)
Solution:
(i) C2H5OH:(2 x 12)+ (5 x 1)
+ (1 x 16) + (1 x 1)=46 g
(ii) KMnO4:(1 x 39)+ (1 x 55) + (4 x
16)=158 g
(iii) K2Cr2O7:(2 x
39)+ (2 x 52) + (7 x 16)=294 g
(iv) C12H22O11:(12
x 12) + (22 x 1) + (11 x 16)=342 g
Evaluate Yourself
3a) Calculate the number of moles
present in 9 g of ethane.
3b) Calculate the number of
molecules of oxygen gas that occupies a volume of 224 ml at 273 K and 3 atm
pressure.
Solution:
(a) Molar mass of ethane, C2H6
= (2 x 12)+ (6 x 1) = 30 g mol-1
n = mass / molar mass = 9 g / 30 g mol-1
= 0.3 mole
(b) At 273 K and 1 atm pressure 1 mole of a gas
occupies a volume of 22.4 L
Therefore,
number of moles of oxygen, that occupies a volume
of 224 ml at 273 K and 3 atm pressure
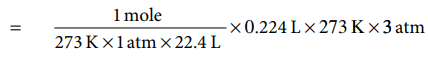
=0.03 mole
1 mole of oxygen contains 6.022 x 1023
molecules
0.03 mole of oxygen contains = 6.022 x 1023 x 0.03
= 1.807 x
1022 molecules of oxygen
Evaluate Yourself
4a) 0.456 g of a metal gives 0.606
g of its chloride. Calculate the equivalent mass of the metal.
4b) Calculate the equivalent mass
of potassium dichromate. The reduction half-reaction in acid medium is,
Cr2O72-
+ 14H+ +6e- → 2 Cr3+ +7H2O
Solution:
(a) Mass of the metal = 0.456 g
Mass of the metal chloride =
0.606 g
0.456 g of the metal combines with 0.15 g of
chlorine.
Mass of the metal that combines with 35.5 g of
chlorine is 0.456/0 .15 × 35.5
= 107.92
g eq–1.
(b) Equivalent mass of a oxidising agent = molar
mass / number of moles of electrons gained by one mole of the reducing agent
= 292.2 g mol-1 / 6 eq
mol-1
= 48.7 g eq-1
Evaluate Yourself
5) A Compound on analysis gave the
following percentage composition C=54.55%, H=9.09%, O=36.36%. Determine the
empirical formula of the compound.
Solution:
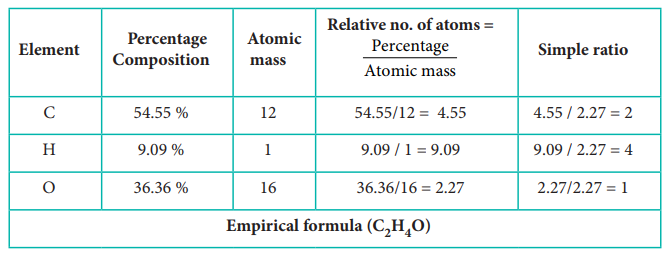
Evaluate Yourself
6) Experimental analysis of a
compound containing the elements x,y,z on analysis gave the following data. x =
32 %, y = 24 %, z = 44 %. The relative number of atoms of x, y and z are 2, 1
and 0.5, respectively. (Molecular mass of the compound is 400 g) Find out.
i) The atomic masses of the
element x,y,z.
ii) Empirical formula of the
compound and
iii) Molecular formula of the
compound.
Solution:
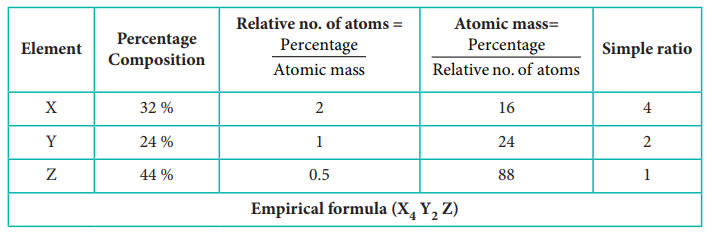
Calculated empirical formula mass = (16 × 4) + (24 × 2) + 88
= 64 + 48 + 88 = 200
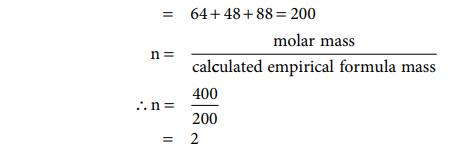
∴ Molecular formula (X4Y2Z)2 = X8Y4Z2
Evaluate Yourself
7) The balanced equation for a
reaction is given below
2x+3y → 4l + m
When 8 moles of x react with 15
moles of y, then
i) Which is the limiting reagent?
ii) Calculate the amount of
products formed.
iii) Calculate the amount of
excess reactant left at the end of the reaction.
Solution:
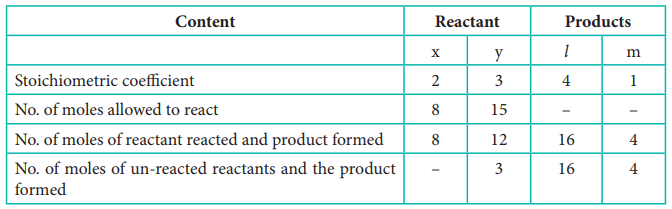
Limiting reagent: x
Product formed: 16 moles of l& 4 moles of m
Amount of excess reactant: 3 moles of y
Evaluate Yourself
8) Balance the following equation
using oxidation number method
As2S3 + HNO3
+ H2O → H3AsO4+H2SO4+NO
Solution:
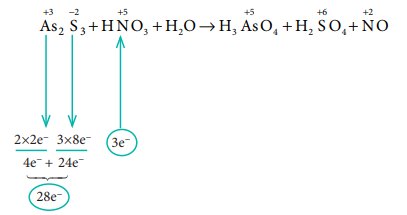
Equate the total no. of electrons in the reactant
side by cross multiplying,
3 As2 S3 +
28 HNO3 + H2O → H3AsO4 + H2SO4
+ NO
Based on reactant side, balance
the products
3 As2 S3 + 28 HNO3
+ H2O → 6 H3AsO4 + 9 H2SO4
+ 28 NO
Product
side:36 hydrogen atoms & 88 oxygen atoms
Reactant
side:28 hydrogen atoms & 74 oxygen atoms
Difference is 8 hydrogen atoms & 4 oxygen atoms
∴ Multiply
H2O molecule on the reactant side by '4'.
Balanced equation is,
3 As2
S3 + 28 HNO3 + 4 H2O → 6 H3AsO4
+ 9 H2SO4 + 28 NO
Related Topics