Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Limiting Reagents
Limiting Reagents:
Earlier, we learnt that the stoichiometry concept is useful in predicting the amount of product formed in a given chemical reaction. If the reaction is carried out with stoichiometric quantities of reactants, then all the reactants will be converted into products. On the other hand, when a reaction is carried out using non-stoichiometric quantities of the reactants, the product yield will be determined by the reactant that is completely consumed. It limits the further reaction from taking place and is called as the limiting reagent. The other reagents which are in excess are called the excess reagents.
Recall the analogy that we used in stoichiometry concept i.e. kesari preparation,
As per the recipe requirement, 2 cups of sugar are needed for every cup of rava. Consider a situation where 8 cups of sugar and 3 cups of rava are available (all other ingredients are in excess), as per the cooking recipe, we require 3 cups of rava and 6 cups of sugar to prepare 18 cups of kesari. Even though we have 2 more cups of sugar left, we cannot make any more quantity of Kesari as there is no rava available and hence rava limits the quantity of Kesari in this case. Extending this analogy for the chemical reaction in which three moles of sulphur are allowed to react with twelve moles of fluorine to give sulfur hexafluoride.
The balanced equation for this reaction is, S + 3F2 → SF6
As per the stoichiometry,
1 mole of sulphur reacts with 3 moles of fluorine to form 1 mole of sulphur hexafluoride and therefore 3 moles of sulphur reacts with 9 moles of fluorine to form 3 moles of sulphur hexafluoride. In this case, all available sulphur gets consumed and therefore it limits the further reaction. Hence sulphur is the limiting reagent and fluorine is the excess reagent. The remaining three moles of fluorine are in excess and do not react.
Urea, a commonly used nitrogen based fertilizer, is prepared by the reaction between ammonia and carbon dioxide as follows.
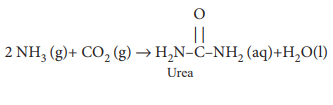
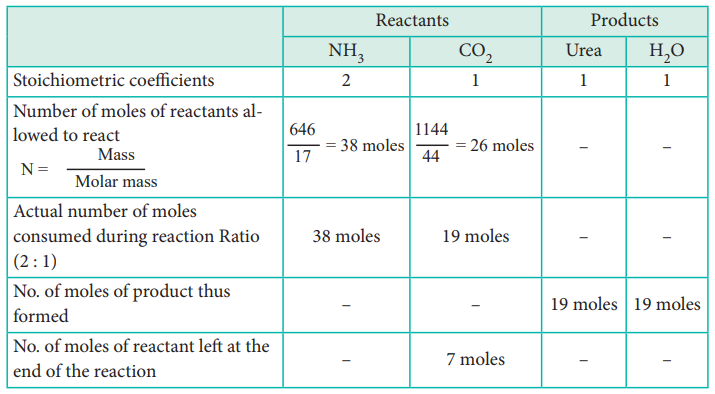
In a process, 646 g of ammonia is allowed to react with 1.144 kg of CO2 to form urea.
1. If the entire quantity of all the reactants is not consumed in the reaction
which is the limiting reagent ?
2. Calculate the quantity of urea formed and unreacted quantity of the excess reagent.
The balanced equation is
2 NH3 + CO2 - > H2NCONH2 + H2O
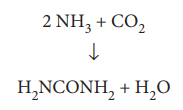
Answer :
1. The entire quantity of ammonia is consumed in the reaction. So ammonia is the limiting reagent. Some quantity of CO2 remains unreacted, so CO2 is the excess reagent.
2. Quantity of urea formed
= number of moles of urea formed × molar mass of urea
= 19 moles × 60 g mol–1
= 1140 g = 1.14 kg
Excess reagent leftover at the end of the reaction is carbon dioxide.
Amount of carbon dioxide leftover
= number of moles of CO2 left over × molar mass of CO2
= 7 moles × 44 g mol–1
= 308 g
Related Topics