with Answers, Solutions - Choose the best answer: Basic Concepts of Chemistry and Chemical Calculations | 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Choose the best answer: Basic Concepts of Chemistry and Chemical Calculations
Basic Concepts of Chemistry and Chemical Calculations
Choose the best answer.
1. 40 ml of methane is completely burnt using 80 ml of oxygen at room temperature The volume of gas left after cooling to room temperature is
(a) 40 ml CO2 gas
(b) 40 ml CO2 gas and 80 ml H2O gas
(c) 60 ml CO2 gas and 60 ml H2O gas
(d) 120 ml CO2 gas
Answer: (a) 40 ml of CO2 gas
Solution:
CH4(g) + 2O2(g) → CO2 (g)+ 2 H2O (l)
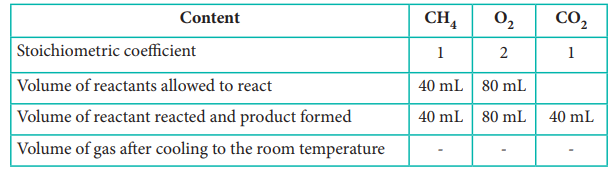
Since the product was cooled to room temperature, water exists mostly as liquid. Hence, option (a) is correct.
2. An element X has the following isotopic composition 200X = 90 %, 199X = 8 % and 202X = 2 %. The weighted average atomic mass of the element X is closest to
(a) 201 u
(b) 202 u
(c) 199 u
(d) 200 u
Answer: (d) 200 u
Solution:
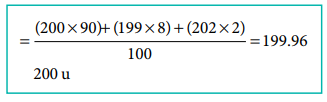
3. Assertion Two mole of glucose contains 12.044 × 1023 molecules of glucose
Reason : Total number of entities present in one mole of any substance is equal to 6.02 × 1022
(a) both assertion and reason are true and the reason is the correct explanation of assertion
(b) both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer: (c) assertion is true but reason is false
Solution:
Correct reason: Total number of entities present in one mole of any substance is equal to 6.022 ×1023.
4. Carbon forms two oxides, namely carbon monoxide and carbon dioxide. The equivalent mass of which element remains constant?
(a) Carbon
(b) oxygen
(c) both carbon and oxygen
(d) neither carbon nor oxygen
Answer: (b) oxygen
Solution:
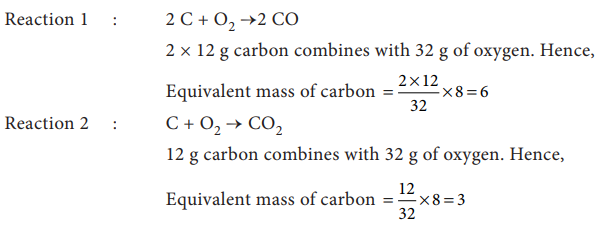
5. The equivalent mass of a trivalent metal element is 9 g eq-1 the molar mass of its anhydrous oxide is
(a) 102 g
(b) 27 g
(c) 270 g
(d) 78 g
Answer: (a) 102 g
Solution:
Let the trivalent metal be M3+
Equivalent mass = mass of the metal / valance factor
9 g eq-1 = mass of the metal / 3 eq
Mass of the metal = 27 g
Oxide formed M2O3 ;
Mass of the oxide = (2 x 27) + (3 x 16)
= 102 g
6.The number of water molecules in a drop of water weighing 0.018 g is
(a) 6.022 × 1026
(b) 6.022 × 1023
(c) 6.022 × 1020
(d) 9.9 × 1022
Answer: (c) 6.022 x 1020
Solution:
Weight of the water drop =0.018 g
No. of moles of water in the drop=Mass of water / molar mass
= 0.018 / 18 = 10-3 mole
No of water molecules present in 1 mole of water= 6.022 x 1023
No. water molecules in one drop of water (10-3 mole)= 6.022 x 1023x 10-3
=6.022 x 1020
7. 1 g of an impure sample of magnesium carbonate (containing no thermally decomposable impurities) on complete thermal decomposition gave 0.44 g of carbon dioxide gas. The percentage of impurity in the sample is
(a) 0 %
(b) 4.4 %
(c) 16 %
(d) 8.4 %
Answer: (c) 16 %
8. When 6.3 g of sodium bicarbonate is added to 30 g of acetic acid solution, the residual solution is found to weigh 33 g. The number of moles of carbon dioxide released in the reaction is
(a) 3
(b) 0.75
(c) 0.075
(d) 0.3
Answer: (c) 0.075
Solution:
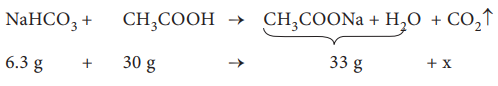
The amount of CO2 released, x = 3.3 g
No. of moles of CO2 released = 3.3 / 44 = 0.075 mol
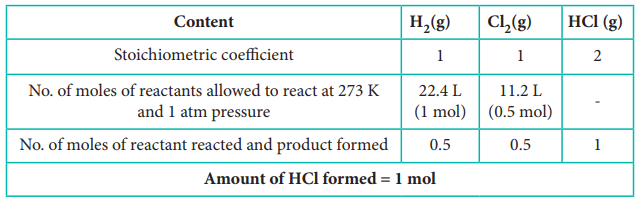
9. When 22.4 litres of H2 (g) is mixed with 11.2 litres of Cl2 (g), each at 273 K at 1 atm the moles of HCl (g), formed is equal to
(a) 2 moles of HCl (g)
(b) 0.5 moles of HCl (g)
(c) 1.5 moles of HCl (g)
(d) 1 moles of HCl (g)
Answer: (d) 1 mole of HCl
Solution:
H2(g) + Cl2(g) → 2 HCl (g)
10. Hot concentrated sulphuric acid is a moderately strong oxidising agent. Which of the following reactions does not show oxidising behaviour?
(a) Cu+ 2H2SO4 → CuSO4 + SO2+2H2O
(b) C+ 2H2SO4 →CO2+2SO2+2H2O
(c) BaCl2 + H2SO4→ BaSO4+2HCl
(d) none of the above
Answer: (c) BaCl2 + H2SO4 → BaSO4+2 HCl
Solution:
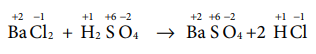
11. Choose the disproportionation reaction among the following redox reactions.
(a) 3Mg (s) + N2 (g) → Mg3N2 (s)
(b) P4 (s) + 3 NaOH+ 3H2O → PH3(g) + 3NaH2PO2 (aq)
(c) Cl2 (g)+ 2KI(aq) → 2KCl(aq) + I2
(d) Cr2O3 (s) + 2Al (s) → Al2O3(s) + 2Cr(s)
Answer: (b) P4 + 3 NaOH + 3 H2O → PH3 + 3 NaH2PO2
Solution:
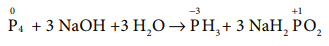
12. The equivalent mass of potassium permanganate in alkaline medium is
MnO4- + 2H2O+3e- → MnO2 + 4OH-
(a) 31.6
(b) 52.7
(c) 79
(d) None of these
Answer: (b) 52.7
Solution:
The reduction reaction of the oxidising agent(MnO4–) involves gain of 3 electrons.
Hence the equivalent mass = (Molar mass of KMnO4)/3 = 158.1/3 = 52.7
13. Which one of the following represents 180g of water?
(a) 5 Moles of water
(b) 90 moles of water
(c) (6.022 x 1023) / 180 molecules of water
(d) 6.022x1024 molecules of water
Answer: (d) 6.022 x 1024 molecules of water
Solution:
No. of moles of water present in 180 g
= Mass of water / Molar mass of water
= 180 g / 18 g mol-1 = 10 moles
One mole of water contains
= 6.022 x 1023 water molecules
10 mole of water contains = 6.022 x 1023 x 10 = 6.022 x 1024 water molecules
14. 7.5 g of a gas occupies a volume of 5.6 litres at 0o C and 1 atm pressure. The gas is
(a) NO
(b) N2O
(c) CO
(d) CO2
Answer: (a) NO
Solution:
7.5 g of gas occupies a volume of 5.6 liters at 273 K and 1 atm pressure Therefore,
the mass of gas that occupies a volume of 22.4 liters
=7.5g / 5.6 L = 30g
Molar mass of NO (14+16) = 30 g
15. Total number of electrons present in 1.7 g of ammonia is
(a) 6.022 x 1023
(b) 6.022 x 1022/1.7
(c) 6.022 x 1024/1.7
(d) 6.022 x 1023/1.7
Answer: (a) 6.022 x 1023
Solution:
No. of electrons present in one ammonia (NH3) molecule (7 + 3) = 10
No of molesof ammonia
=Mass/Molar mass
=1.7g/ 17 gmol-1
=0.1 mol
No of molecules present in 0.1 mol of ammonia
= 0.1x 6.022 x1025= 6.022 x 1022
No of elec . n trons present in mol of ammonia
=10 x 6.022 x1022 = 6.022x1023
16. The correct increasing order of the oxidation state of sulphur in the anions SO42-, SO32- , S2O42-, S2O62- is
(a) SO32- < SO42- < S2O42-< S2O62-
(b) SO42- < S2O42- < S2O62-< SO32-
(c) S2O42- < SO32- < S2O62-< SO42-
(d) S2O62- < S2O42- < SO42-< SO32-
Answer: (c) S2O42- < SO32- < S2O62- < SO42-
Solution:
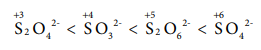
17. The equivalent mass of ferrous oxalate is
(a) molar mass of ferrous oxalate/1
(b) molar mass of ferrous oxalate/2
(c) molar mass of ferrous oxalate/3
(d) none of these
Answer: (c) molar mass of ferrous oxalate / 3
Solution:
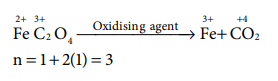
18. If Avogadro number were changed from 6.022 × 1023 to 6.022 x 1020, this would change
(a) the ratio of chemical species to each other in a balanced equation
(b) the ratio of elements to each other in a compound
(c) the definition of mass in units of grams
(d) the mass of one mole of carbon
Answer: (d) the mass of one mole of carbon
Solution:
The mass of one mole of carbon
19. Two 22.4 litre containers A and B contains 8 g of O2 and 8 g of SO2 respectively at 273 K and 1 atm pressure, then
(a) Number of molecules in A and B are same
(b) Number of molecules in B is more than that in A.
(c) The ratio between the number of molecules in A to number of molecules in B is 2:1
(d) Number of molecules in B is three times greater than the number of molecules in A.
Answer: (c) the ratio between the number of molecules in A to number of molecules in B is 2:1
Solution:
No, of moles of oxygen=8 g/32 g
=0.25 moles of oxygen
No. of moles of sulphur dioxide=8 g / 64 g
=0.125 moles of sulphur dioxide
Ratio between the no. of molecules=0.25: 0.125
=2:1
20. What is the mass of precipitate formed when 50 ml of 8.5 % solution of AgNO3 is mixed with 100 ml of 1.865 % potassium chloride solution?
(a) 3.59 g
(b) 7 g
(c) 14 g
(d) 28 g
Answer: (a) 3.59 g
Solution:
AgNO3 + KCl → KNO3 + AgCl
50 mL of 8.5 % solution contains 4.25 g of AgNO3
No. of moles of AgNO3 present in 50 mL of 8.5 % AgNO3 solution
=Mass / Molar mass
=4.25 / 170
=0.025 moles
Similarly, No of moles of KCl present in 100 mL of 1.865 % KCl solution
=1.865 / 74.5
=0.025 moles
So total amount of AgCl formed is 0.025 moles (based on the stoichiometry)
Amount of AgCl present in 0.025 moles of AgCl
=no. of moles x molar mass
=0.025 x 143.5 = 3.59 g
21. The mass of a gas that occupies a volume of 612.5 ml at room temperature and pressure (250 c and 1 atm pressure) is 1.1g. The molar mass of the gas is
(a) 66.25 g mol-1
(b) 44 g mol-1
(c) 24.5 g mol-1
d) 662.5 g mol-1
Answer: (b) 44 g mol-1
Solution:
No. of moles of a gas that occupies a volume of 612.5 mL at room temperature and pressure (25 0 C and 1 atm pressure)
=612.5 × 10-3 L / 24.5 Lmol-1
=0.025 moles
We know that,
Molar mass =Mass / no. of moles
=1.1 g /0.025 mol = 44 g mol-1
22. Which of the following contain same number of carbon atoms as in 6 g of carbon-12.
(a) 7.5 g ethane
(b) 8 g methane
(c) both (a) and (b)
(d) none of these
Answer: (c) both (a) & (b)
Solution:
No. of moles of carbon present in 6 g of C-12 = Mass / Molar mass
=6/12 = 0.5 moles = 0.5 × 6.022 × 1023 carbon atoms. No. of moles in 8 g of methane = 8 / 16 = 0.5 moles
=0.5 × 6.022 × 1023 carbon atoms.
=2 × 0.25 × 6.022 × 1023 carbon atoms.
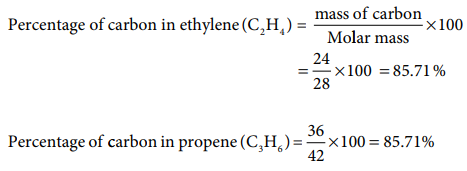
23. Which of the following compound(s) has /have percentage of carbon same as that in ethylene (C2H4)
(a) propene
(b) ethyne
(c) benzene
(d) ethane
Answer: (a) propene
Solution:
24. Which of the following is/are true with respect to carbon -12.
(a) relative atomic mass is 12 u
(b) oxidation number of carbon is +4 in all its compounds.
(c) 1 mole of carbon-12 contain 6.022 × 1022 carbon atoms.
(d) all of these
Answer: (a) relative atomic mass is 12 u
Solution:
(a) relative atomic mass of C-12 is 12 u
25. Which one of the following is used as a standard for atomic mass.
(a) 6C12
(b) 7C12
(c) 6C13
(d) 6C14
Answer: (a) 6C12
Related Topics