Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Empirical Formula and Molecular Formula
Empirical
Formula and Molecular Formula
Elemental analysis of a compound gives the mass percentage
of atoms present in the compound. Using the mass percentage, we can determine
the empirical formula of the compound. Molecular formula of the compound can be
arrived at from the empirical formula using the molar mass of the compound.
Empirical formula of a compound is the formula written
with the simplest ratio of the number of different atoms present in one
molecule of the compound as subscript to the atomic symbol. Molecular formula
of a compound is the formula written with the actual number of different atoms
present in one molecule as a subscript to the atomic symbol.
Let us understand the empirical formula by considering
acetic acid as an example.
The molecular formula of acetic acid is C2H4O2
The ratio of C : H : O is 1 : 2 : 1 and hence the
empirical formula is CH2O.
Determination of Empirical Formula from Elemental Analysis Data :
Step 1: Since the composition
is expressed in
percentage, we can consider the
total mass of the compound as 100 g and the percentage values of individual
elements as mass in grams.
Step 2: Divide the mass of each element by
its atomic mass. This gives the relative number of moles of various elements in
the compound.
Step 3: Divide the value of relative
number of moles obtained in the step 2 by the smallest number of them to get
the simplest ratio.
Step 4: (only if necessary) in case the
simplest ratios obtained in the step 3 are not whole numbers then they may be
converted into whole number by multiplying by a suitable smallest number.
Example:
1. An acid found in tamarinds on analysis shows the following
percentage composition: 32 % Carbon; 4 % Hydrogen; 64 % Oxygen. Find the
empirical formula of the compound.
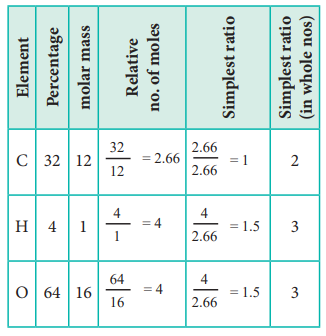
The empirical formula is C2H3O3
2. An organic compound present in vinegar has 40 % carbon,
6.6 % hydrogen and 53.4 % oxygen. Find the empirical formula of the compound.
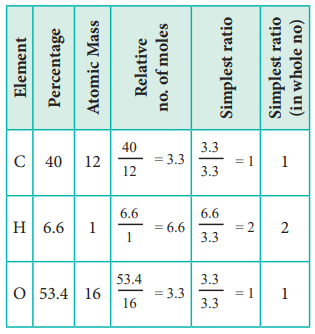
The empirical formula is CH2O
Molecular formula of a compound is a whole number multiple
of the empirical formula. The whole number can be calculated from the molar
mass of the compound using the following expression
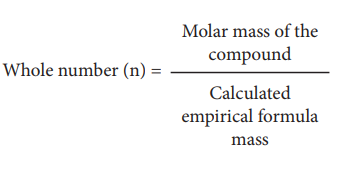
Calculation of Molecular Formula from Empirical Formula:
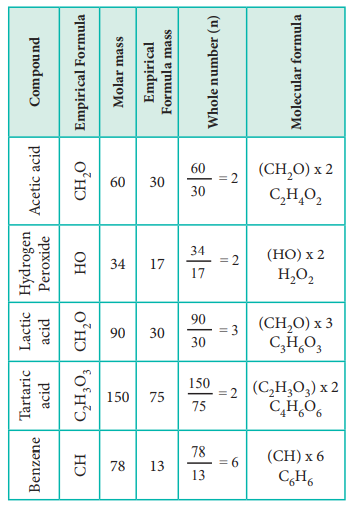
Let us understand the calculations of molecular mass from
the following example.
Two organic compounds, one present in vinegar (molar mass:
60 g mol–1), another one present in sour milk (molar mass 90 g mol–1)
have the following mass percentage composition. C-40%, H-6.6% ; O-53.4%. Find
their molecular formula.
Since both compounds have same mass percentage
composition, their empirical formula are the same as worked out in the example problem no 2. Empirical formula is CH2O.
Calculated empirical formula mass
(CH2O) = 12 + (2…1) + 16 = 30 g mol–1.
Formula for the compound present in vinegar
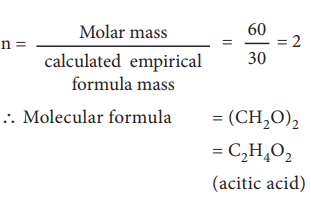
Calculation of molecular formula for the compound present
in sour milk.
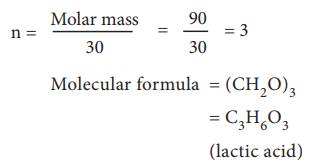
Related Topics