Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Determination of Empirical Formula from Elemental Analysis Data
Determination of Empirical Formula from Elemental Analysis Data :
Step 1: Since the composition is expressed in percentage, we can consider the total mass of the compound as 100 g and the percentage values of individual elements as mass in grams.
Step 2: Divide the mass of each element by its atomic mass. This gives the relative number of moles of various elements in the compound.
Step 3: Divide the value of relative number of moles obtained in the step 2 by the smallest number of them to get the simplest ratio.
Step 4: (only if necessary) in case the simplest ratios obtained in the step 3 are not whole numbers then they may be converted into whole number by multiplying by a suitable smallest number.
Example:
1. An acid found in tamarinds on analysis shows the following percentage composition: 32 % Carbon; 4 % Hydrogen; 64 % Oxygen. Find the empirical formula of the compound.
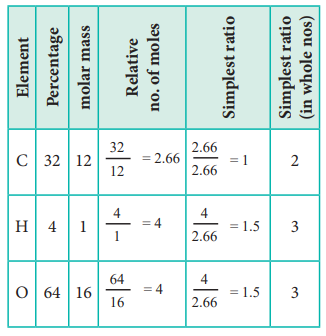
The empirical formula is C2H3O3
2. An organic compound present in vinegar has 40 % carbon, 6.6 % hydrogen and 53.4 % oxygen. Find the empirical formula of the compound.
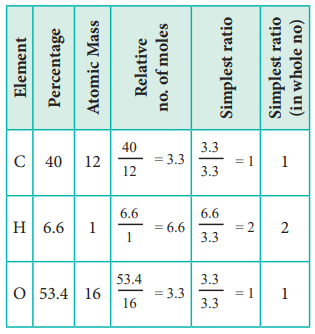
The empirical formula is CH2O
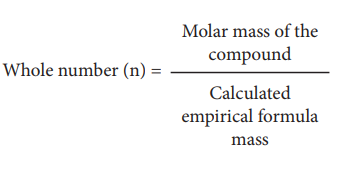
Molecular formula of a compound is a whole number multiple of the empirical formula. The whole number can be calculated from the molar mass of the compound using the following expression
Related Topics