Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Calculation of Molecular Formula from Empirical Formula
Calculation of Molecular Formula from Empirical Formula:
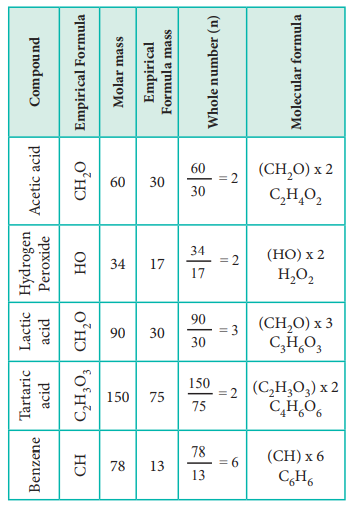
Let us understand the calculations of molecular mass from the following example.
Two organic compounds, one present in vinegar (molar mass: 60 g mol–1), another one present in sour milk (molar mass 90 g mol–1) have the following mass percentage composition. C-40%, H-6.6% ; O-53.4%. Find their molecular formula.
Since both compounds have same mass percentage composition, their empirical formula are the same as worked out in the example problem no 2. Empirical formula is CH2O. Calculated empirical formula mass (CH2O) = 12 + (2…1) + 16 = 30 g mol–1.
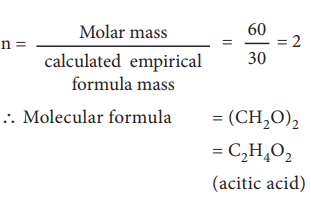
Formula for the compound present in vinegar
Calculation of molecular formula for the compound present in sour milk.
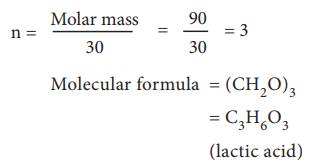
Related Topics