Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Molecularity of the reaction
Molecularity of the reaction
Molecularity is defined as the number of atoms
or molecules taking part in an elementary step leading to a chemical reaction.
The overall chemical reaction may consist of many elementary steps. Each
elementary reaction has its own molecularity which is equal to number of atoms
or molecules participating in it. If the reaction takes place in more than one
step there is no molecularity for the overall reaction. However molecularity
and order are identical for elementary reaction (one step).
There are
many differences between the concepts of order and molecularity.
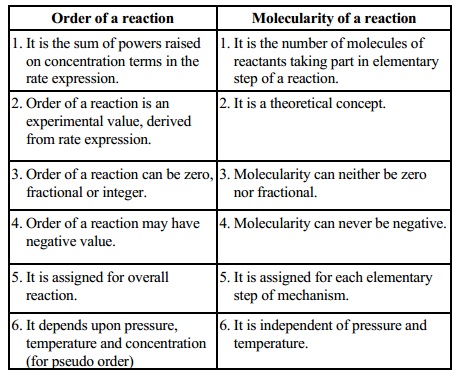
Order
of a reaction
1. It is the sum of powers raised on
concentration terms in the rate expression.
2. Order of a reaction is an
experimental value, derived from rate expression.
3. Order of a reaction can be zero,
fractional or integer.
4. Order of a reaction may have negative
value.
5. It is assigned for overall reaction.
6. It depends upon pressure, temperature
and concentration (for pseudo order)
Molecularity
of a reaction
1. It is the number of molecules of
reactants taking part in elementary step of a reaction.
2.It is a theoretical concept.
3.Molecularity can neither be zero nor
fractional.
4.Molecularity can never be negative.
5.It is assigned for each elementary
step of mechanism.
6.It is independent of pressure and
temperature.
Rate determining step
Most of the chemical reactions occur by multistep reactions. In the
sequence of steps it is found that one of the steps is considerably slower than
the others. The overall rate of the reaction cannot be lower in value than the
rate of the slowest step. Thus in a multistep reaction the experimentally
determined rate corresponds to the rate of the slowest step. Thus the step
which has the lowest rate value among the other steps of the reaction is called
as the rate determining step (or) rate limiting step.
Consider the reaction,
2A + B -- > C + D going by two steps like,
A
+ B - K1-- > C + Z -
(1)step(slow)
Z
+ A ---K2 -- > D -
(2)Step(fast)
2A+B
-- > C+ D
Here, the overall rate of the reaction
corresponds to the rate of the first step which is the slow step and thus, the
first step is called as the rate determining step of the reaction. In the above
reaction, the rate of the reaction depends upon the rate constant k1 only. The
rate of 2nd step doesn't contribute experimentally determined
overall rate of the reaction.
Related Topics