Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Classification of rates based on the order of the reaction
Classification of rates based on the order of the reaction
The rate law for a reaction must be determined
by experiment. Usually the order of the reaction determined experimentally does
not coincide with the stoichiometric coefficients of the reactants or products
in the balanced chemical equation. Each reaction proceeds by a rate value
determined by the rate constant and initial concentrations of the reacting
species. Rate constant values differ for different `order' reactions even if
concentrations are maintained the same. Therefore chemical reactions are
classified according to its rate of chemical transformation which inturn depend
on the order of the reaction. Let us consider a general rate equation such as
rate = k[A]p [B]q
Total order is p + q and order with respect to A is p and with respect
to B in q respectively.
Zero order reaction
A reactant whose concentration does not affect the reaction rate is
called as zero order reaction,
rate law is,
rate = k[A]0
-d[A]/dt
= k or k =[A]0-[A]t / t
Examples of zero order reaction is
H 2 (g) + Cl2 (g) -hv- > < -- 2HCl(g)
The first order reaction
when aqueous solution of NH4NO2 is warmed it decomposes rapidly to H2O
and N2.
NH4NO2 -- > 2H2O + N2
This reaction goes by first order manner rate constant k is given by
K
= 2.303/t . log(Vinf/Vinf-Vt) sec-1
![]() V¥ and Vt are volume of N2
collected at room temperature and 1 atm when a fixed amount of NH4NO2
decomposes at t = ¥ (after
completion of reaction) and at any time `t'.
V¥ and Vt are volume of N2
collected at room temperature and 1 atm when a fixed amount of NH4NO2
decomposes at t = ¥ (after
completion of reaction) and at any time `t'.
Second
order reaction
A reaction is said to be second order if its
reaction rate is determined by the variation of two concentration terms or
square of a single concentration term.
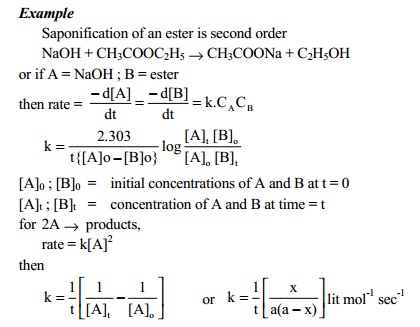
Third order
reactions
A
reaction is said to be third order if its rate is determined by the variation of three concentration terms.
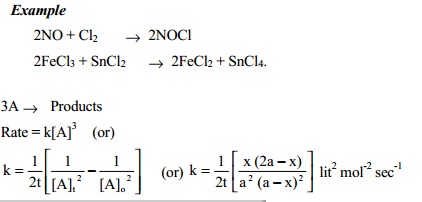
A
second (or) third (or) any other high order reaction can be experimentally
followed in an easy way by reducing the overall order to first order type by
adopting pseudo order conditions. In this method, excluding the concentration of one of
reactants, concentrations of all other reactant are kept in excess (at least 10
times) of the concentration of one of the reactant whose concentrations are to
be varied to study the changes in the rate.

Related Topics